Enalapril maleate oral solution and preparation method thereof
A technology of oral solution and enalapril, which is applied in the field of oral solution powder composition containing enalapril and its salts, can solve problems such as instability of enalapril maleate oral solution, achieve accurate dosage, Convenience to take and increase compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Stability Evaluation
[0020] Enalapril maleate powder formula: mix enalapril maleate with lactose, sucrose or xylitol, and prepare 10% (w / w) enalapril maleate by granulation Lithium powder was used to investigate the stability under different conditions. The prescription is shown in Table 1.
[0021] compound prescription 1 prescription 2 prescription 3 Enalapril Maleate (mg) 150 150 150 Xylitol (mg) 1350 Lactose (mg) 1350 Sucrose (mg) 1350
[0022] Enalapril maleate was slowly dissolved in 12% sodium bicarbonate solution. Place the stabilizer (lactose, sucrose or xylitol) in a mixer and slowly spray the enalapril maleate solution into the stabilizer for about 20 minutes with low agitation. Dry the wetted material in an oven at 60°C for at least 4 hours. The dried granules were passed through a 40 mesh screen. The particles were collected, an appropriate amount was sealed in a PET bottle, and the ...
Embodiment 2
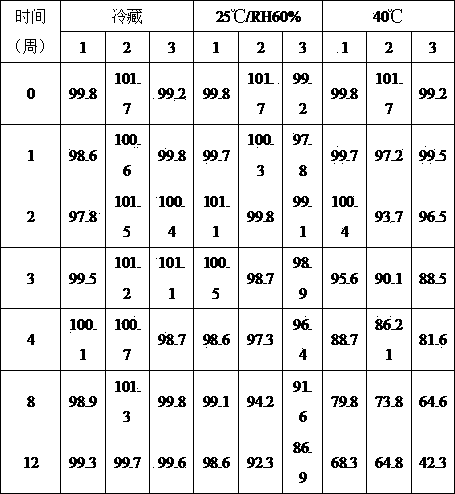
[0029] Example 2: Evaluation of Mixing Uniformity
[0030] Enalapril maleate powder composition was prepared by dry mixing method to investigate the effect of mixing time on drug content uniformity. The formula is shown in Table 4.
[0031] compound prescription 4 prescription 5 Enalapril Maleate (mg) 150 150 Xylitol (mg) 900 890 Colloidal silica (mg) 10
[0032] Increase in proportion as prescribed in Table 4 (150mg enalapril maleate, 900mg xylitol / vial) or (150mg enalapril maleate, 890mg xylitol, 10mg colloidal silica / vial) Weigh each component, directly mix and sieve, take 3 point samples from different positions, and measure the content uniformity after mixing. The results are shown in Table 5:
[0033] Table 5 Results of the effect of colloidal silica on the mixing uniformity of enalapril maleate powder
[0034]
[0035] Although colloidal silica has been reported to reduce the stability of enalapril (Resend et al., Stability ...
Embodiment 3
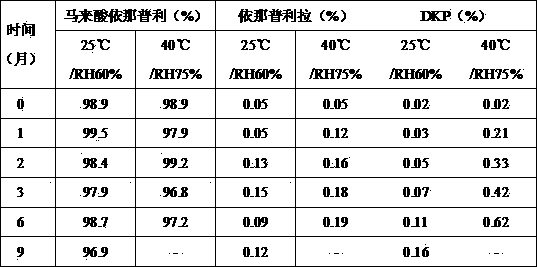
[0036] Example 3: Stability Evaluation
[0037] The samples prepared according to recipe 5 in Example 2 were placed under the conditions of 25°C / RH60% and 40°C / RH75%. Samples were taken at different time points, and the content of enalapril maleate and related substances were determined by HPLC / UV. The results are shown in Table 6.
[0038] Table 6 Stability evaluation results of enalapril maleate powder
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com