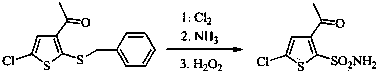Method for preparing intermediate thiophene sulfonamide of brinzolamide
A technology of thiophene sulfonamide and phene sulfonamide, which is applied in the field of preparation of brinzolamide intermediate thiophene sulfonamide, can solve the problems of unfavorable environmental protection, heavy odor, and high risk of butyl lithium, so as to reduce the harm to the human body and optimize the preparation The method, the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
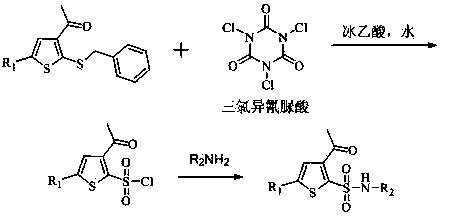
[0027] Add 210g of 3-acetyl-5-chloro-2-(benzylmercapto)thiophene and 1500g of acetonitrile into a 5L four-neck flask, stir, add 320g of glacial acetic acid and 200g of water, control the temperature between 0-15°C, and batch Add 241.6g of trichloroisocyanuric acid, and complete the addition in about 30 minutes, keep the reaction at 0-15°C, and monitor the reaction by TLC until the raw materials disappear. Water bath at 35-45°C, concentrate under reduced pressure to remove the solvent, add 1500g of 25% ethyl acetate petroleum ether solution, stir, filter, and evaporate the filtrate to dryness under reduced pressure to obtain 182.7g of light yellow oil, which is 3-acetyl-5- Chloro-2-thiophenesulfonyl chloride.
[0028] Dilute the above-mentioned sulfonyl chloride with 105g of ethyl acetate, control the temperature at 0-15°C, add it dropwise to 420g of ammonia water, stir the reaction until the sulfonyl chloride disappears, lower the temperature to about 0°C, and stir for crystal...
Embodiment 2
[0030] Add 210g of 3-acetyl-5-chloro-2-(benzylmercapto)thiophene and 1500g of ethyl acetate to a 5L four-neck flask, stir, add 320g of glacial acetic acid and 200g of water, and control the temperature between 0-15°C. Add 241.6 g of trichloroisocyanuric acid in batches, complete the addition in about 30 minutes, keep the reaction at 0-15°C, and monitor the reaction by TLC until the raw materials disappear. Water bath at 40-50°C, concentrate under reduced pressure to remove the solvent, add 1500g of 25% ethyl acetate petroleum ether solution, stir, filter, and evaporate the filtrate to dryness under reduced pressure to obtain 180.3g of light yellow oily substance, which is 3-acetyl-5- Chloro-2-thiophenesulfonyl chloride.
[0031] Dilute the above-mentioned sulfonyl chloride with 105g of ethyl acetate, control the temperature at 0-15°C, add it dropwise to an aqueous solution containing 46g of methylamine, stir the reaction until the sulfonyl chloride disappears, lower the temper...
Embodiment 3
[0033] Add 184.4g of 3-acetyl-2-(benzylmercapto)thiophene and 1500g of dichloromethane into a 5L four-neck flask, stir, add 320g of glacial acetic acid and 200g of water, control the temperature between 0-15°C, and add in batches 241.6g of trichloroisocyanuric acid was added in about 30 minutes, kept at 0-15°C for reaction, and monitored by TLC until the raw materials disappeared. Water bath at 30-35°C, concentrate under reduced pressure to remove the solvent, add 1500g of 25% ethyl acetate petroleum ether solution, stir, filter, and evaporate the filtrate to dryness under reduced pressure to obtain 160.3g of light yellow oil, which is 3-acetyl-2- Thiophenesulfonyl chloride.
[0034] Dilute the above-mentioned sulfonyl chloride with 105g of ethyl acetate, control the temperature at 0-15°C, add it dropwise to 420g of ammonia water, stir the reaction until the sulfonyl chloride disappears, lower the temperature to about 0°C, and stir for crystallization for 1h. Filter, wash wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com