Palmatine hydrochloride crystal form C as well as preparation method thereof and application thereof in medicament composition or health-care product
A kind of palmatine hydrochloride crystal, palmatine hydrochloride technology, applied in the field of palmatine hydrochloride crystal form C and its preparation, can solve the problems such as no patent or literature report of palmatine hydrochloride crystal form found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation method 1 of palmatine hydrochloride crystal type C sample:
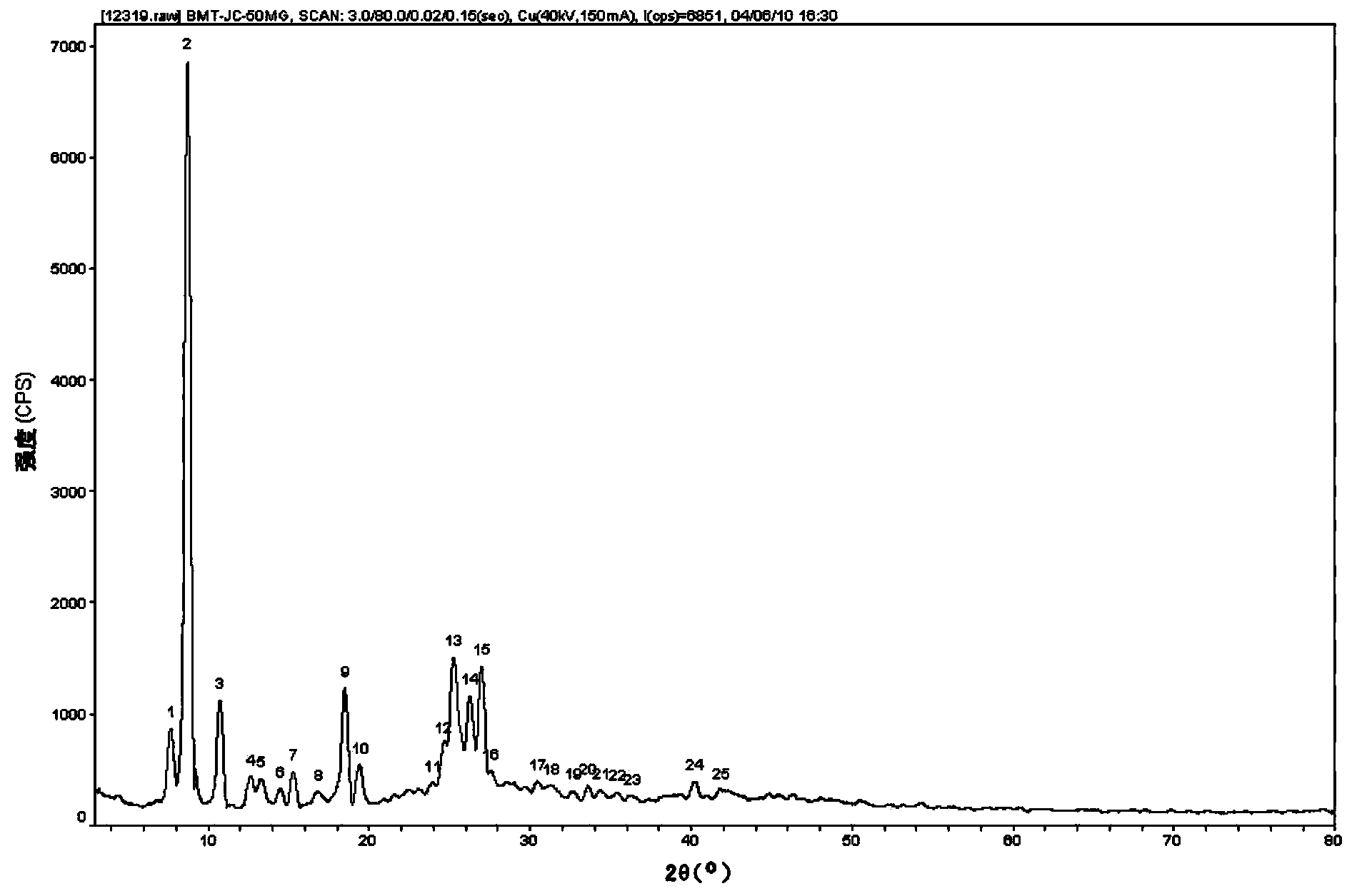
[0047] The preparation method of palmatine hydrochloride crystal type C sample is characterized in that 200 mg of palmatine hydrochloride raw material is completely dissolved at 20°C by using 40mL chloroform as a solvent, and the solvent is removed under vacuum pressure at 35°C to prepare palmatine hydrochloride crystal type C Sample 1. The powder X-ray diffraction pattern of the sample is as figure 1 As shown, the infrared absorption spectrum is shown as figure 2 shown, and the DSC spectrum as image 3 shown.
[0048] Preparation method 2 of palmatine hydrochloride crystal type C sample:
[0049] The preparation method of palmatine hydrochloride crystal type C sample is characterized in that 200mg of palmatine hydrochloride raw material is completely dissolved at 20°C using 15mL of methanol as a solvent, and the solvent is removed under vacuum pressure at 40°C to prepare palmatine hydrochlori...
Embodiment 2
[0070] Palmatine hydrochloride new crystal type C absorption characteristics and blood concentration characteristics in rats:
[0071] SD male rats with a body weight of 190-220 g were reared under conventional feeding conditions, free to drink water, and after fasting for 12 hours, the drug was administered by intragastric administration at 200 mg / kg. Before and after administration, 0.083, 0.167, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8 (h), take about 0.5ml of blood from the retroocular vein, and centrifuge at 4500rpm for 10min. Take 200 μl of plasma, add 400 μl of methanol, vortex for 2 minutes, centrifuge at 13400 rpm for 10 minutes, take the supernatant layer, and dry it with nitrogen. Add 100 μl mobile phase (acetonitrile: 0.05% H 3 PO 4 =27:73), vortexed for 1 min, centrifuged at 13400 rpm for 1 min, and 40 μl of the supernatant was taken for HPLC detection. The HPLC detection system is Aligent1200 high-performance liquid chromatography system, and the chromatographic colum...
Embodiment 3
[0075] Preparation of pharmaceutical tablets:
[0076] Mix palmatine hydrochloride crystal type C prepared in the above example 1 with lactose, starch, low-substituted hydroxypropyl cellulose, and microcrystalline cellulose, and add 1% sodium hydroxymethyl cellulose solution to make a soft material. Sieve and granulate the soft material, dry the wet granules, sieve, add talcum powder and magnesium stearate, mix evenly, and compress into tablets to obtain pharmaceutical tablets with various dosages as follows.
[0077] Formulation of table 2 drug tablet
[0078]
[0079] Preparation of drug capsules:
[0080] Mix palmatine hydrochloride crystal type C prepared in the above example 1 with lactose, starch and microcrystalline cellulose evenly, add 1% sodium hydroxymethyl cellulose solution to make soft material, sieve and granulate the soft material, The wet granules are dried, sieved, then added magnesium stearate and mixed uniformly, and packed into capsules to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com