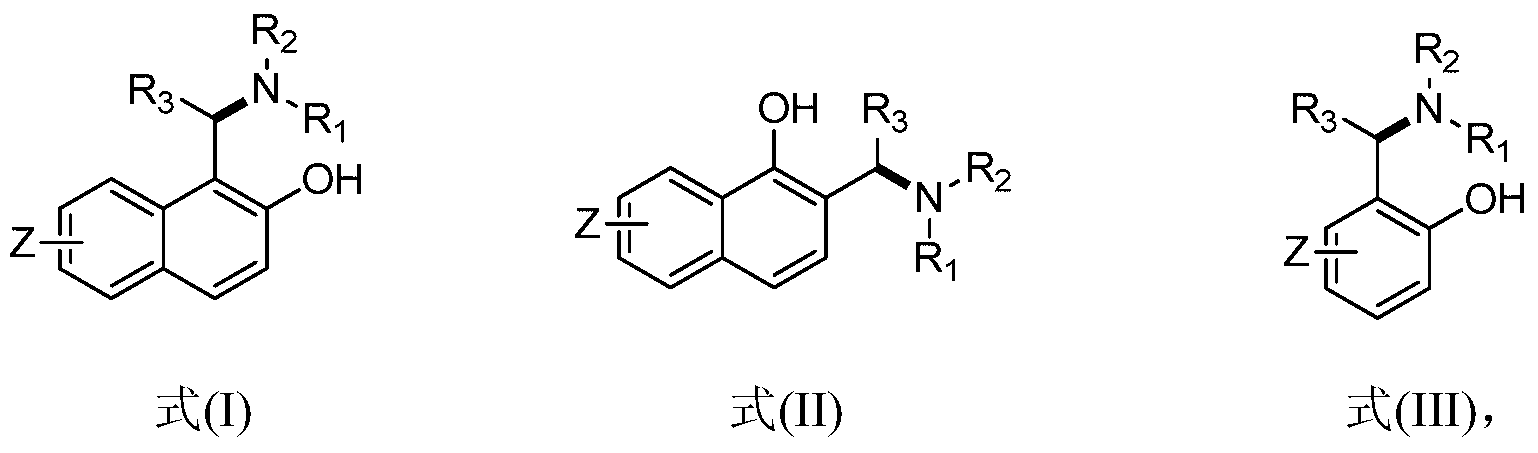
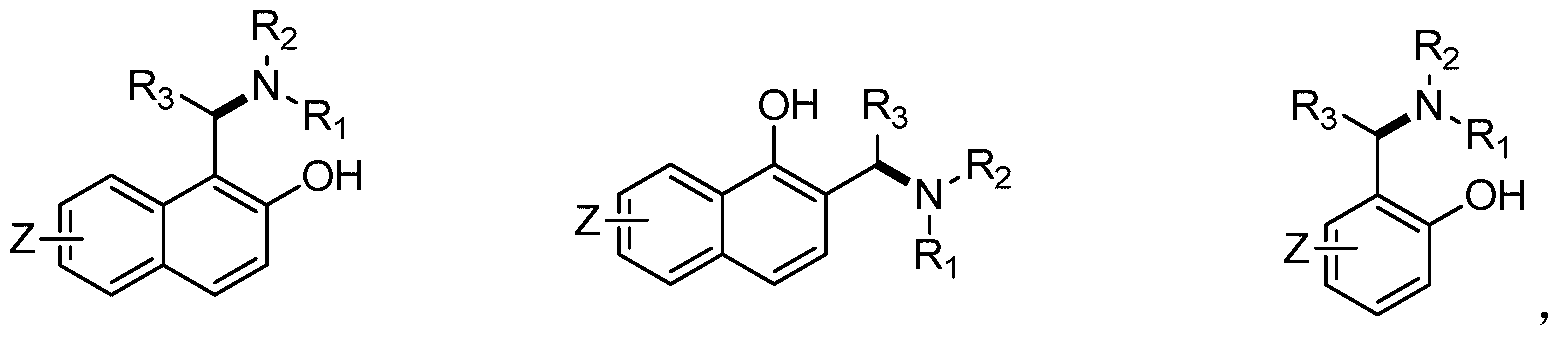
Application of chiral aminophenol ligand to asymmetric synthesis of efavirenz
A technology of efavirenz and aminophenol, applied in the field of asymmetric synthesis of chiral aminophenol ligands in efavirenz, can solve the problems of chiral resolution and application lag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
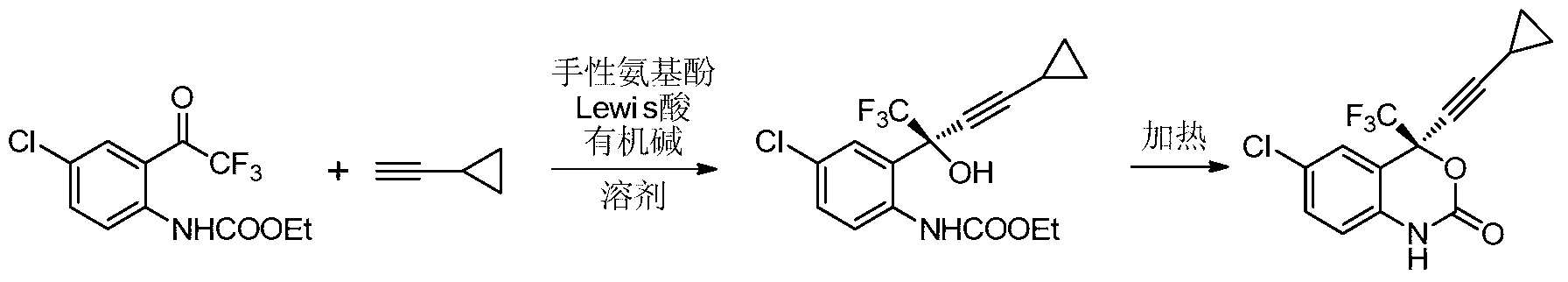
[0053] (S)-6-Chloro-4-cyclopropynyl-4-trifluoromethyl-1,4-dihydro-2H-1,3-benzoxazin-2-one (Efavirenz) asymmetric synthesis
[0054]Add (R)-1-(phenyl(pyrrolidin-1-yl)methyl)naphthalene-2-alcohol (23mg, 0.75mmol) and zinc p-toluenesulfonate (20mg, 0.5mmol) to the sealed tube, and extract Ventilate, add cyclopropylacetylene (0.4mL), triethylamine (0.14mL, 10mmol), stir for 3h, add (4-chloro-2-trifluoroacetylphenyl) ethyl carbamate (30mg, 0.1 mmol), the reaction was stirred at room temperature for 24h. Organic solvent such as ethyl acetate (EA) was transferred, washed with 6M hydrochloric acid, washed with saturated sodium chloride, and dried over anhydrous sodium sulfate. Column chromatography, (S)-(4-chloro-2-(4-cyclopropyl-1,1,1-trifluoro-2-hydroxybut-3-yn-2-yl)phenyl)carbamic acid Ethyl ester 35.5mg, yield 96%, ee value 81%. (The separation here is only for data analysis, and this step of separation may not be performed during actual synthesis)
[0055] 1 H NMR (300MHz, ...
Embodiment 2
[0065] (S)-6-Chloro-4-cyclopropynyl-4-trifluoromethyl-1,4-dihydro-2H-1,3-benzoxazin-2-one (Efavirenz) asymmetric synthesis
[0066] Add (R)-1-(phenyl(pyrrolidin-1-yl)methyl)naphthalene-2-alcohol (68mg, 2.25mmol) and zinc p-toluenesulfonate (61mg, 1.5mmol) to the sealed tube, and extract Ventilate, add cyclopropylacetylene (0.4mL), triethylamine (0.14mL, 10mmol), stir for 8h, add (4-chloro-2-trifluoroacetylphenyl) ethyl carbamate (30mg, 0.1 mmol), the reaction was stirred at room temperature for 24h. Add water to quench, extract with EA, wash with 6M hydrochloric acid, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. After concentration, add toluene to reflux reaction for 4 hours to obtain 31.1 mg of efavirenz with a yield of 98% and an ee value of 91%.
Embodiment 3
[0068] (S)-6-Chloro-4-cyclopropynyl-4-trifluoromethyl-1,4-dihydro-2H-1,3-benzoxazin-2-one (Efavirenz) asymmetric synthesis
[0069] Add (R)-2-(phenyl(pyrrolidin-1-yl)methyl)naphthalene-1-ol (68mg, 2.25mmol) and zinc p-toluenesulfonate (61mg, 1.5mmol) to the sealed tube, and extract Ventilate, add cyclopropylacetylene (0.4mL), triethylamine (0.14mL, 10mmol), transfer to 40°C oil bath, stir for 3h, add (4-chloro-2-trifluoroacetylphenyl) carbamic acid Ethyl ester (30mg, 0.1mmol), stirred at room temperature for 24h. Add water to quench, extract with EA, wash with 6M hydrochloric acid, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. After concentration, add toluene to reflux reaction for 4 hours to obtain 31.5 mg of efavirenz with a yield of 94% and an ee value of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com