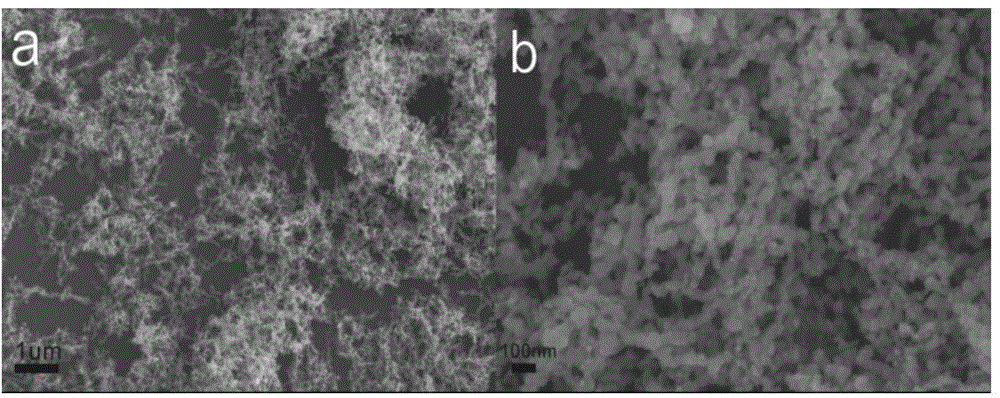
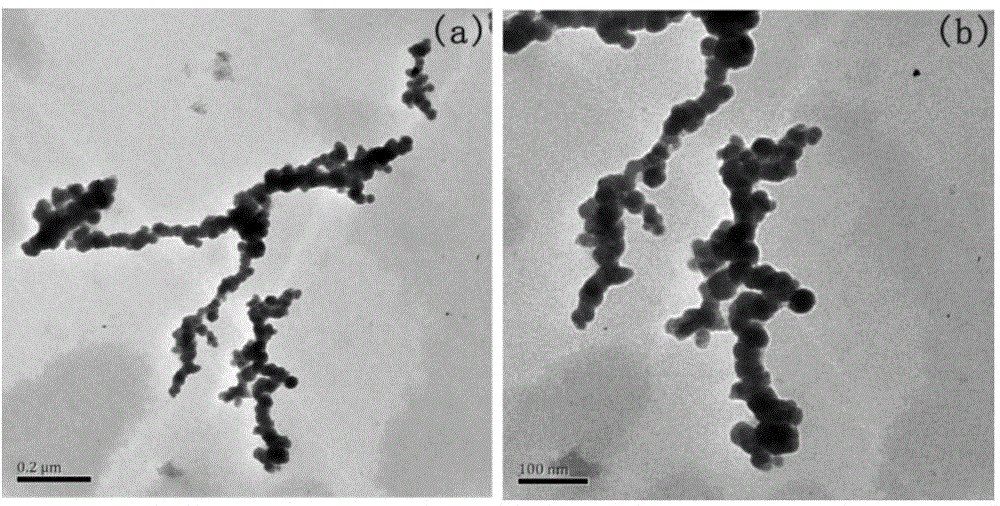
Cobalt-based amorphous nanometer wave-absorbing material and synthetic method of cobalt-based amorphous nanometer wave-absorbing material
A technology of nano-wave absorbing materials and synthesis methods, applied in the fields of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of high surface atomic ratio, small nanoparticle size, large hysteresis loss, etc. , to achieve the effect of good reproducibility, slowing down the reaction rate and large hysteresis loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Prepare 250ml10mM CoCl 2 Aqueous solution Weigh 594.8 mg of cobalt chloride hexahydrate and dissolve it in a small amount of ultrapure water. After ultrasonic dispersion at room temperature for 1 min, it is diluted to a concentration of 10 mM.
[0025] (2) Add the solution obtained in step (1) to a 500mL three-necked round-bottomed flask, place the three-necked flask in a constant temperature heating magnetic stirrer, connect the hollow glass tube to the left side and insert it below the liquid level of the solution, The other end of the glass tube is connected to the rubber tube connected to nitrogen; the middle port is connected to the reflux condenser, and the upper end is connected to a glass cock; Nitrogen gas for 0.5 ~ 1.0h.
[0026] (3) Prepare 25ml of 2.5M NaBH 4 The aqueous solution weighs 2.365g and dissolves it in ultrapure water, and the dilution concentration is 2.5M.
[0027] (4) After the reaction in step (2) is completed, use a syringe to get the ...
Embodiment 2
[0035] (1) Prepare 250ml10mM CoCl 2 Aqueous solution Weigh 594.8 mg of cobalt chloride hexahydrate and dissolve it in a small amount of ultrapure water. After ultrasonic dispersion at room temperature for 1 min, it is diluted to a concentration of 10 mM.
[0036](2) Add the solution obtained in step (1) to a 500mL three-necked round-bottomed flask, place the three-necked flask in a constant temperature heating magnetic stirrer, connect the hollow glass tube to the left side and insert it below the liquid level of the solution, The other end of the glass tube is connected to the rubber tube connected to nitrogen; the middle port is connected to the reflux condenser, and the upper end is connected to a glass cock; Nitrogen gas for 0.5 ~ 1.0h.
[0037] (3) Prepare 25ml of 2.5M NaBH 4 The aqueous solution weighs 2.365g and dissolves it in ultrapure water, and the dilution concentration is 2.5M.
[0038] (4) After the reaction in step (2) is completed, use a syringe to get the N...
Embodiment 3
[0045] (1) Prepare 250ml10mM CoCl 2 Aqueous solution Weigh 594.8 mg of cobalt chloride hexahydrate and dissolve it in a small amount of ultrapure water. After ultrasonic dispersion at room temperature for 1 min, it is diluted to a concentration of 10 mM.
[0046] (2) Add the solution obtained in step (1) to a 500mL three-necked round-bottomed flask, place the three-necked flask in a constant temperature heating magnetic stirrer, connect the hollow glass tube to the left side and insert it below the liquid level of the solution, The other end of the glass tube is connected to the rubber tube connected to nitrogen; the middle port is connected to the reflux condenser, and the upper end is connected to a glass cock; Nitrogen gas for 0.5 ~ 1.0h.
[0047] (3) Prepare 50ml of 2.5M NaBH 4 The aqueous solution weighs 4.730g and dissolves it in ultrapure water, and the dilution concentration is 2.5M.
[0048] (4) After the reaction in step (2) is completed, use a syringe to get the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com