Memantine hydrochloride sustained-release pills and preparation method thereof
A technology of memantine hydrochloride and sustained-release pills is applied in sugar-coated pills, pharmaceutical formulations, and devices that make medicines into special physical or taking forms, etc. Burst release, guaranteed curative effect, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: In order to study the impact of the isolation layer on the dissolution of the sustained-release capsule under high humidity conditions, two kinds of pellets were prepared, namely, drug-containing pellet A without isolation layer (or isolation coating layer) and drug-containing pellet B with isolation layer , and then poured into the capsules to form corresponding slow-release capsules A and slow-release capsules B, and put them into a closed container with a relative humidity of 92.5% to investigate the dissolution results in 5 and 10 days.
[0042] One, the preparation of sustained-release capsule A
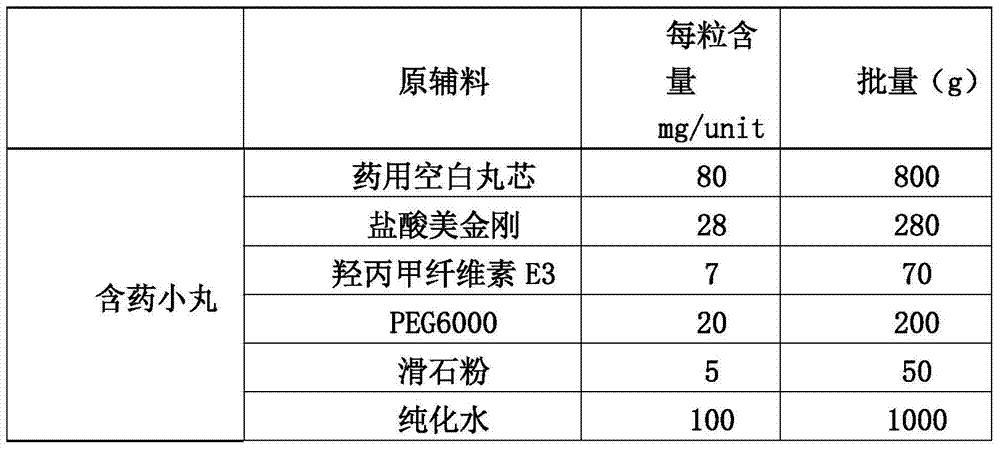
[0043] (1) Preparation of drug-containing pellets of memantine hydrochloride:
[0044]
[0045] 1.1 Weigh hypromellose E3 into a container filled with purified water at 80°C to 90°C, stir to disperse, then add cold purified water, and stir while adding, to obtain I;
[0046] 1.2 Put polyethylene glycol 6000 and memantine hydrochloride in a container, add...
Embodiment 2
[0083] Embodiment 2: the preparation method of memantine hydrochloride sustained-release capsules:
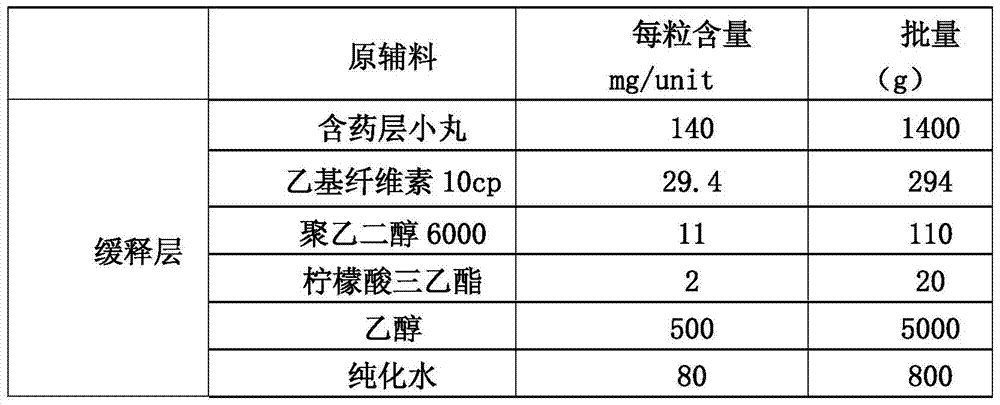
[0084] (1): Preparation of main drug layer pellets containing memantine hydrochloride (i.e. drug-containing pellets):
[0085]
[0086] 1.1 Weigh hydroxypropyl cellulose and add it to a container filled with purified water at 80°C to 90°C, stir to disperse, then add cold purified water, and stir while adding to obtain I.
[0087] 1.2 Put polyethylene glycol and memantine hydrochloride in a container, add purified water to dissolve, and obtain II.
[0088] 1.3 Then add (I) into (II), stir evenly, then add talcum powder while stirring, stir to disperse the talc powder evenly, pass through an 80-mesh sieve, keep stirring gently with the agitator, and obtain the drug-containing coating solution for later use.
[0089] 1.4 Put the blank pellet core into the material cylinder of the fluidized bed by manual feeding, start heating, and when the temperature of the material is kept a...
Embodiment 3
[0104] Embodiment 3: the preparation method of memantine hydrochloride sustained-release capsules
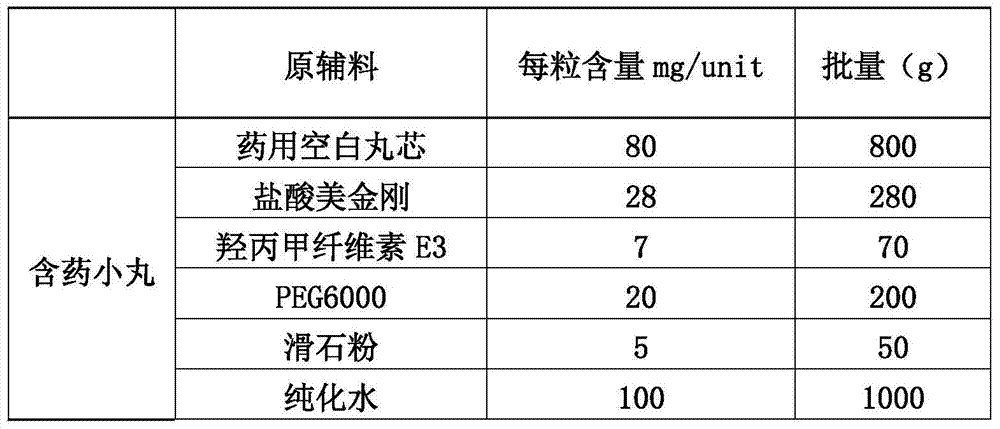
[0105] (1) Preparation of main drug layer pellets:
[0106]
[0107] 1.1 Weigh hydroxypropyl cellulose and add it to a container filled with purified water at 80°C to 90°C, stir to disperse, then add cold purified water, and stir while adding to obtain I.
[0108] 1.2 Put polyethylene glycol and memantine hydrochloride in a container, add purified water to dissolve, and obtain II.
[0109] 1.3 Then add (I) into (II), stir evenly, then add talcum powder while stirring, and stir to disperse the talcum powder evenly. Pass through an 80-mesh sieve and keep stirring gently with the agitator to obtain a drug-containing coating solution for later use.
[0110] 1.4 Put the blank pellet core into the material cylinder of the fluidized bed by manual feeding, start heating, and when the temperature of the material is kept above 35°C, turn on the atomization pressure (1.6BAR), adjust t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com