Preparing method for human immune globulin
A human immunoglobulin and protein technology, applied in the field of human immunoglobulin preparation, can solve the problems of long cycle, low yield and purity, and achieve the effects of shortened production cycle, high product purity and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Adopt the present invention to prepare human immunoglobulin
[0033] 1. Plasma collection: collect raw plasma with high titer, and its rabies surface antibody titer should be ≥ 8IU / ml;
[0034] 2. Separation of components: Dilute the above-mentioned raw plasma with 0.14mol / L sodium chloride solution, adjust the pH to 7.10±0.04 with acetic acid buffer solution with a pH of 4.0, cool down to 0°C and start spraying 95% of it below -15°C ethanol, so that the final ethanol concentration reaches 12% (v / v), control the temperature at -2.5±0.5°C, adjust the pH to 7.10±0.04 with acetic acid buffer, stir for 2 hours, add diatomaceous earth, and press filter to separate the above Clear liquid A, control pressure ≤ 0.1MPa during filter press;
[0035] 3. Precipitation filter press: Continue to spray 95% ethanol below -15°C into the above supernatant A to make the final ethanol concentration reach 20% (v / v), control the temperature at -5.0±0.5°C, and use pH Adjust th...
Embodiment 2
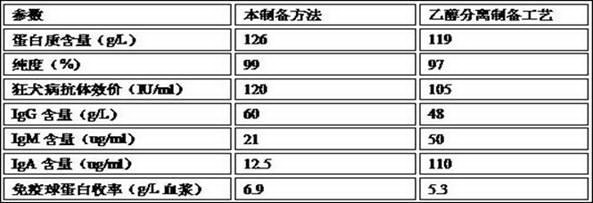
[0046] Example 2: Comparing the rabies patient immunoglobulin prepared by this process with the traditional ethanol separation preparation process, the main parameters of the obtained rabies patient immunoglobulin product:
[0047]
[0048] Therefore, the quality and yield of the product prepared by the method are better than those prepared by traditional ethanol separation.
Embodiment 3
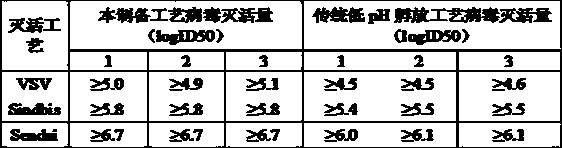
[0049] Embodiment 3: The inactivated virus process of this preparation method is compared with the low pH hatching inactivated virus process
[0050]
[0051] Therefore, the virus inactivation method used in this process is more effective than the traditional low pH virus inactivation method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com