Lithium zirconate-cladded lithium-rich positive material for lithium ion battery and preparation method thereof
A lithium-rich positive electrode material, lithium-ion battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem of unsatisfactory charge and discharge rate, specific capacity charging cycle performance, increase the manufacturing cost of lithium-ion batteries, and irreversible capacity of materials Attenuation and other issues, to achieve good cycle performance, high rate charge and discharge capacity, simple and flexible preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
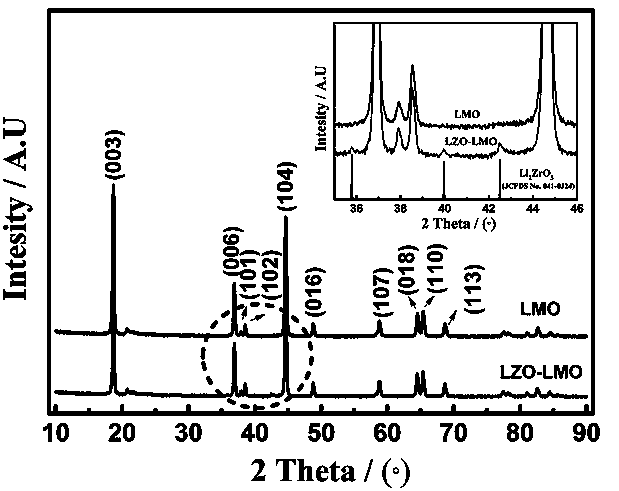
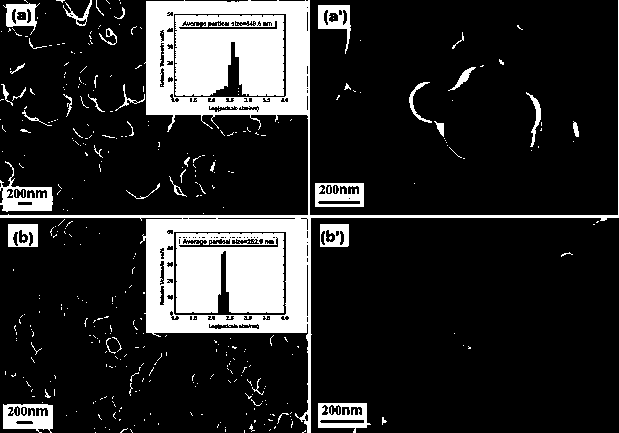
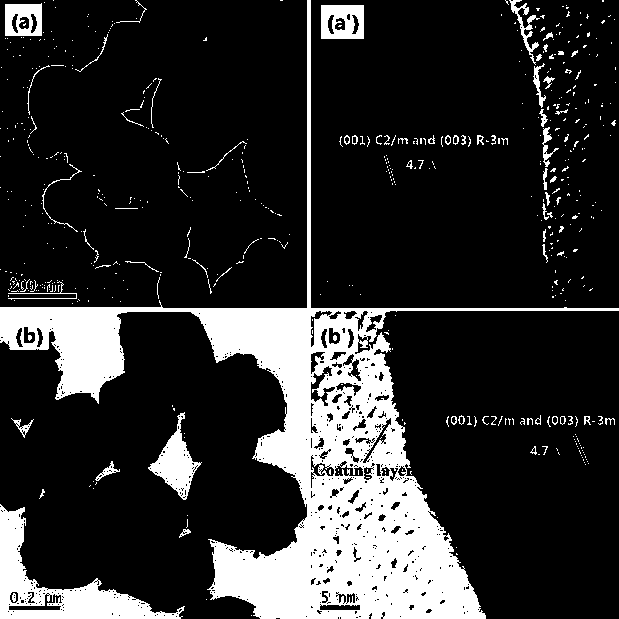
[0029] In this embodiment, the lithium-rich positive electrode material of the lithium-ion battery coated with lithium zirconate, with 0.4Li 2 MnO 3 0.6LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 As the core of the composite particles of lithium-rich cathode materials, and with Li 2 ZrO 3 As the shell of composite particles of lithium-rich cathode materials, 0.4Li 2 MnO 3 0.6LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 Abbreviated as LMO, where Li 2 ZrO 3 It is referred to as LZO, that is, LMO is coated with LZO to form an LMO-LZO core-shell structure of composite particles with a particle size of 50nm~300nm, forming a LMO-LZO nanocomposite material, in which LZO is in the LMO-LZO lithium-rich cathode material. The weight percent content is 2wt%.
[0030] In this embodiment, the preparation method of the lithium-rich positive electrode material of the lithium-ion battery coated with lithium zirconate comprises the following steps:
[0031] a. Preparation of LMO: According to the chemical element ...
Embodiment 2
[0038] This embodiment is basically the same as Embodiment 1, especially in that:
[0039] In this embodiment, the preparation method of the lithium-rich positive electrode material of the lithium-ion battery coated with lithium zirconate comprises the following steps:
[0040] a. Preparation of LMO: According to the chemical element composition of LMO, the stoichiometric ratio of cobalt acetate, manganese acetate, nickel acetate and lithium acetate was added to the same beaker and dissolved in absolute ethanol to form a metal salt mixture, and keep lithium acetate In the metal salt mixture, according to the excess molar content of 5%, citric acid is used as a chelating agent, dissolved in absolute ethanol, and then the absolute ethanol solution containing citric acid is kept at a constant temperature in an oil bath at 70°C, and then The metal salt mixed solution containing excess lithium acetate is slowly added dropwise into the absolute ethanol solution containing citric aci...
Embodiment 3
[0045] This embodiment is basically the same as the previous embodiment, and the special features are:
[0046] In this embodiment, the preparation method of the lithium-rich positive electrode material of the lithium-ion battery coated with lithium zirconate comprises the following steps:
[0047] a. Preparation of LMO: According to the chemical element composition of LMO, the stoichiometric ratio of cobalt acetate, manganese acetate, nickel acetate and lithium acetate was added to the same beaker and dissolved in absolute ethanol to form a metal salt mixture, and keep lithium acetate In the metal salt mixture, according to the excess molar content of 5%, citric acid is used as a chelating agent, dissolved in absolute ethanol, and then the absolute ethanol solution containing citric acid is kept at a constant temperature in an oil bath at 70°C, and then The metal salt mixed solution containing excess lithium acetate is slowly added dropwise into the absolute ethanol solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Maximum discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com