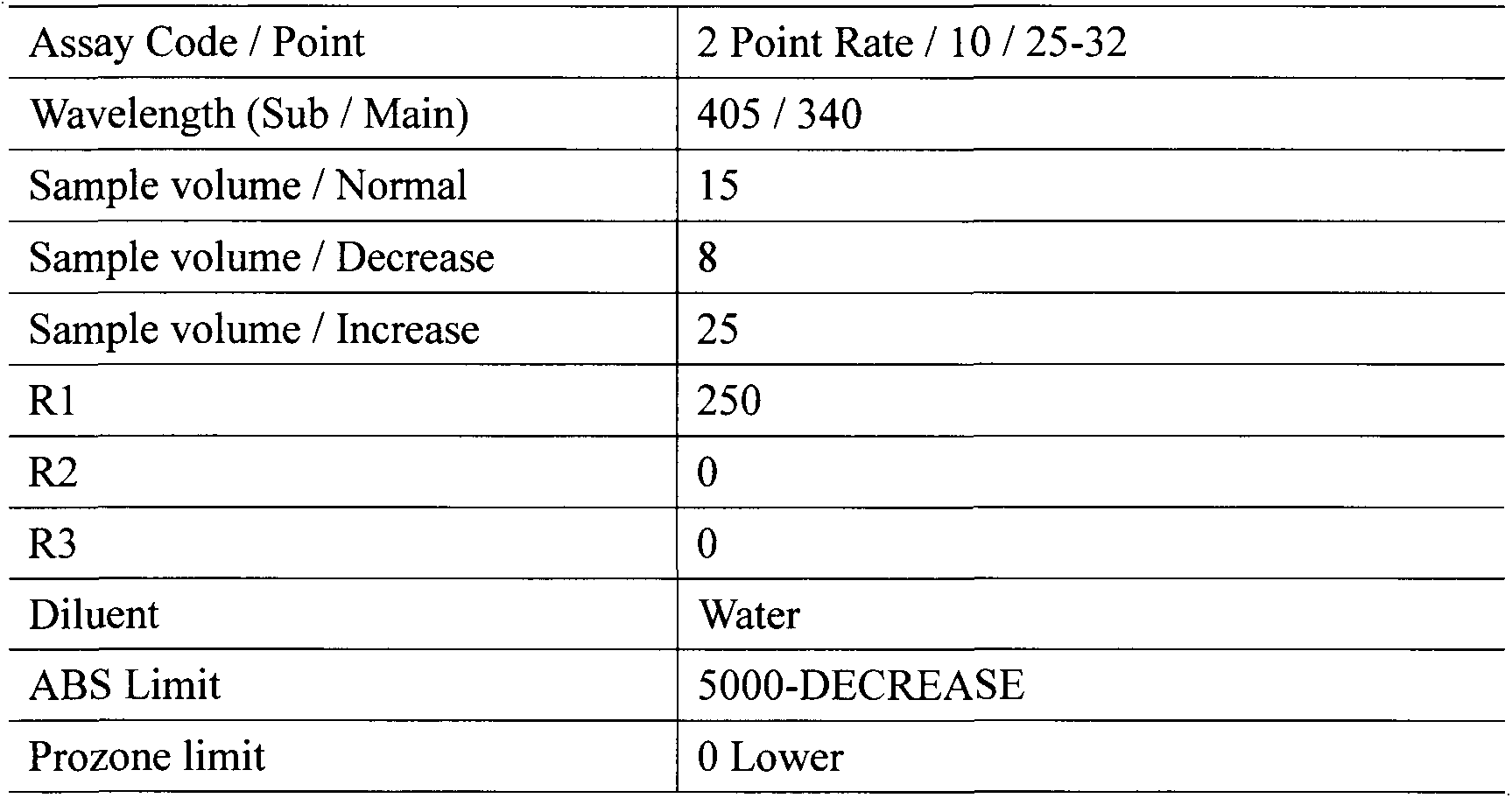
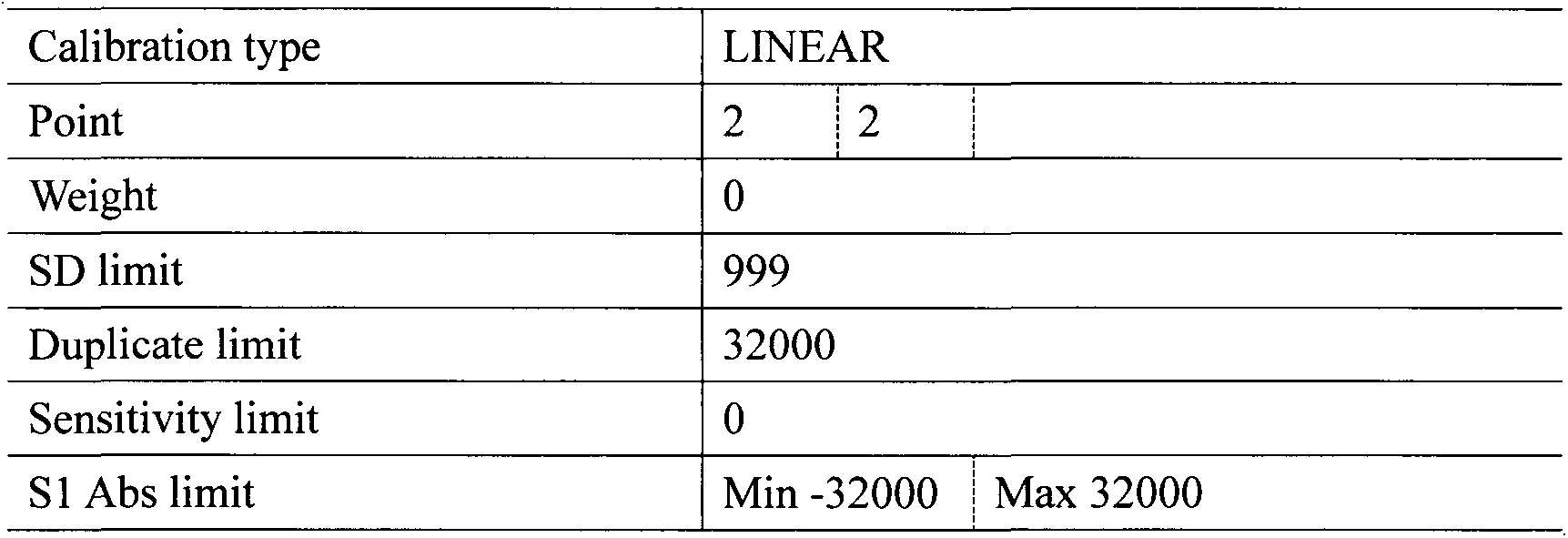
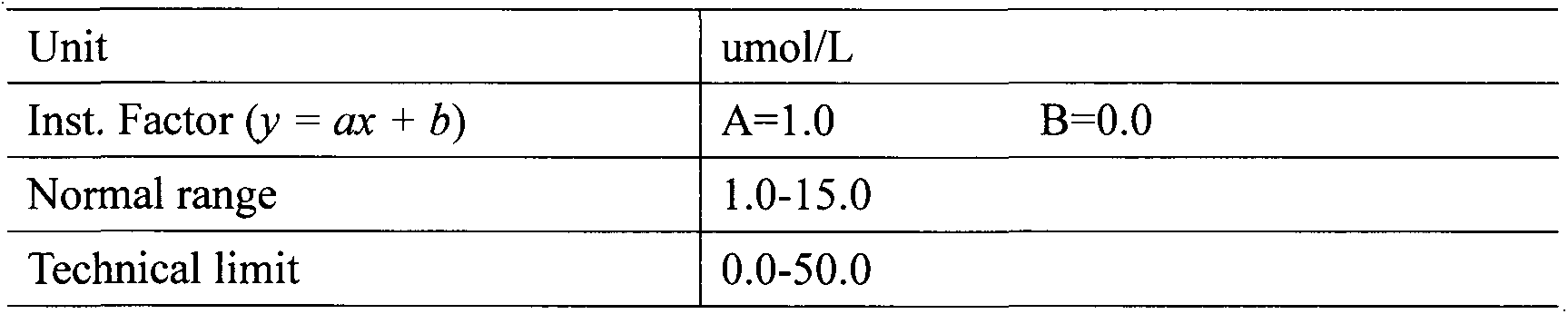
Homocysteine detection using fusion enzymes
A homocysteine, fusion enzyme technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve problems such as difficult control, restricting catalytic efficiency, etc., to improve accuracy and stability, and improve detection. horizontal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Expression and purification of embodiment 1 fusion enzyme protein
[0026]The fusion enzyme protein uses a flexible linker peptide (GGGGS) 2 , applied to the fusion of homocysteine S-methyltransferase (EC2.1.1.10) and adenosylhomocysteinase (EC3.3.1.1), and adenosylhomocysteinase (EC3.3.1.1) and methionine adenosyltransferase fusion (EC2.5.1.6), the construction method of the fusion gene is consistent:
[0027] Design the upstream primer with the EcoR I restriction site for the first enzyme gene, and the downstream primer with GGGGS and the 5' end partial sequence of the second enzyme gene; design the 3' end partial sequence with GGGGS and the first enzyme gene An upstream primer, and a downstream primer with a Xhol I restriction site for the second enzyme gene fragment. Using the American ABI gradient PCR instrument (Veriti TM 96), the amplification system and reaction conditions of the two are the same (total volume 100ul, 10ul of 10*PCR buffer, 10ul of 100umo / L ...
Embodiment 2
[0039] Embodiment 2 Utilizes the comparison of the detection accuracy of the reagent prepared by fusion enzyme and common enzyme cycle reagent
[0040] Reagents prepared using fusion enzymes contain the following components:
[0041] Reagent components
Dosage per liter
Phosphate buffer, pH7.6, 37°C
100mM
26.5mM
7.7mM
ATP
2mM
3ku
H-G-Aase
10ku
A-E-Mase
10ku
0.5%
alpha-ketoglutarate
7.5mM
60mM
0.5mM
Glycerin
100g
reduced coenzyme NADH
0.29mM
2ku
[0042] The contrast reagent is a commercially available general enzyme cycle reagent, which is calibrated with a prepared homocysteine standard solution with a concentration of 15.1 μM. The detection sample is a prepared linear sample, and the concentrations of h...
Embodiment 3
[0054] Embodiment 3 utilizes the comparison of the thermal stability of the reagent prepared by fusion enzyme and common enzyme cycle reagent
[0055] The liquid dual-reagent reagent prepared by fusion enzyme contains the following components:
[0056] Reagent 1:
Dosage per liter
Tris buffer, pH9.1, 20.0°C
100mM
26.5mM
15.0mM
0.5mM
alpha-ketoglutarate
7.5mM
Triton X-100
2.0g
2.0g
reduced coenzyme NADH
0.29mM
7ku
3ku
[0057] Reagent 2:
Dosage per liter
HEPES buffer, pH7.4, 20.0°C
50mM
60mM
ATP
10mM
15.0mM
PEG2000
0.5g
Tween-20
3.0g
1.0g
H-E-Aase
50ku
A-G-Mase
70ku
[0059] The co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com