Asymmetric perylene bisimide compounds with star structures and preparing method thereof
A technology of star structure and perylene imide, which is applied in the direction of organic chemistry, etc., to achieve the effects of enhanced solvency, novel structure, and easy charge transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of unsymmetrical peryleneimide monoanhydride:
[0032] Add 7.84g (20mM) of 3,4,9,10-perylenetetracarboxylic anhydride into 100ml of 5% potassium hydroxide solution, heat the solution to hydrolyze the ring-opening of the anhydride, and when the pH value of the solution is stabilized between 9.5 and 11.5, add to the system Start to add 20wt% dilute hydrochloric acid aqueous solution dropwise until all the products in the solution just precipitate out. Stop the reaction and cool to room temperature, filter with a funnel and wash with deionized water until the filtrate becomes neutral, then dry to obtain an intermediate product, the color of which is purple.
[0033] Add 4.9ml (3.875g, 30mM) of 2-ethylhexylamine and 4.485g (10mM) of the product from the previous step to a mixed solution of 50ml of isopropanol and water at a volume ratio of 7:3, and react at a low temperature of 0°C for 4 hours , and then heated up to 90°C to react for 2 hours, acidified the sys...
Embodiment 2
[0036] The preparation of the asymmetric perylene imide compound of star structure:
[0037]4,4', 4"-triaminotriphenylamine (290.25mg, 1mmol), asymmetric peryleneimide monoacid anhydride (2.014g, 4mmol) and imidazole (10g, 146mmol) were added to the three-necked flask at one time, and Under the protection of nitrogen, heat to 100°C to melt imidazole into a liquid state. After stirring and reacting at this temperature for 1 hour, react at 130°C for 2 hours and then raise the temperature to 160°C for 3 hours. After the reaction is completed, the system is cooled to room temperature. Pour the product into a mixed solution of methanol and 2mol / L dilute hydrochloric acid aqueous solution to precipitate out, then extract the organic phase with chloroform several times and rotary evaporate to obtain the product, use methanol / chloroform (1:40) as the eluent to separate through the column Dried to obtain the target product with a star structure. The mass of the obtained product was 1.0...
Embodiment 3
[0040] The preparation of the asymmetric perylene imide compound of star structure:
[0041] Add 4,4',4"-triaminotriphenylamine (290.25mg, 1mmol) and asymmetric peryleneimide monoacid anhydride (2.52g, 5mmol) into 20ml m-cresol solvent, heat to 90°C under nitrogen protection ℃, stirred and reacted at this temperature for 2 hours, then added 2ml of isoquinoline, raised the temperature to 160°C for 4 hours, and then raised the temperature to 200°C for 6 hours. After the reaction, the system was cooled to room temperature, and the product was poured into methanol and 2mol / L dilute hydrochloric acid aqueous solution to precipitate, and then extract the organic phase with chloroform for many times and then rotary evaporate to obtain the product, use methanol / chloroform (1:40) as the eluent to separate through the column and dry to obtain a star-shaped structure Target product. The mass of the product obtained was 0.8 g.
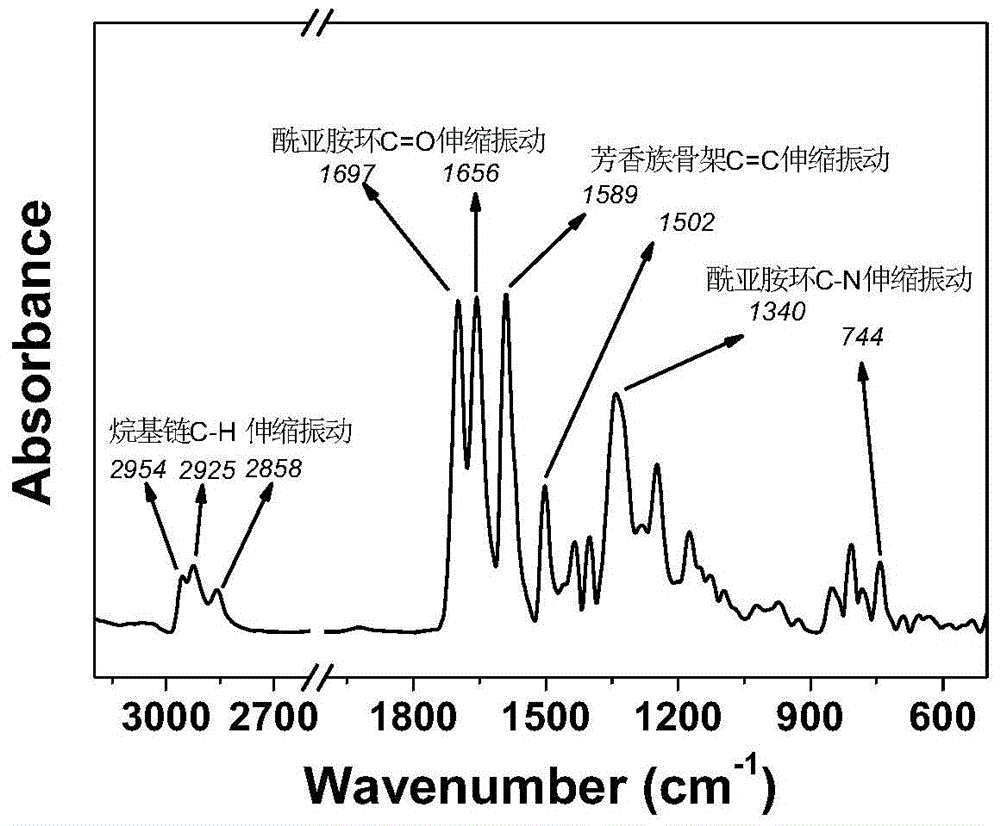
[0042] Productive rate 45.8%; Infrared (KBr tablet): 2954-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com