Method for screening DOX (doxorubicin)-nephrotoxicity-resistant active substances through three-color fluorescence labeling
A fluorescent labeling and anti-doxorubicin technology, which is applied in the direction of fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of single information, not conducive to the comprehensive evaluation of the effect of compound cells, and missing screening, so as to reduce the dosage, screening and evaluation The results are comprehensive and rigorous, and the effect of eliminating experimental errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
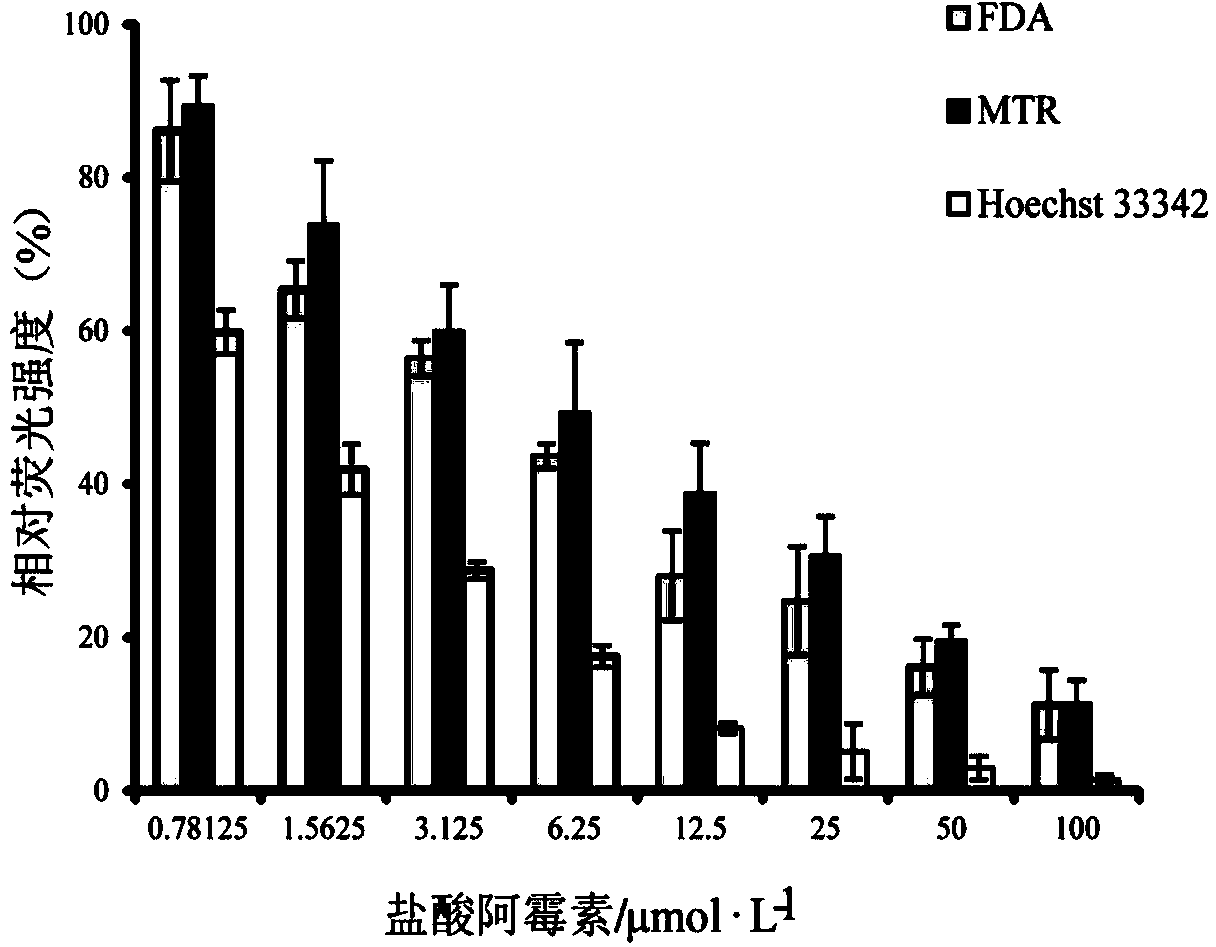
[0056] Example 1 Three-color fluorescent probe labeling kidney cells to evaluate the damage effect of doxorubicin hydrochloride
[0057] HK-2 cells were labeled with three fluorescent probes FDA, MTR, and Hoechst33342, and the damage degree of doxorubicin hydrochloride to HK-2 cells was reflected by the change of fluorescence intensity.
[0058] HK-2 cells were inoculated in 96-well plates at 5000 cells / well, and incubated in a cell culture incubator for 24 hours to allow the cells to adhere to the wall; the old culture solution was sucked off, and 90 μL of culture solution (high-glucose DMEM basal medium) was added to each well. Then add 10 μL of culture solution containing 7.8125, 15.625, 31.25, 62.5, 125, 250, 500 and 1000 μmol / L doxorubicin hydrochloride in sequence; a solvent control group (containing 0.1% DMSO) was also set up in the experiment, and each group had 3 replicate wells, and incubated 24h; Aspirate the old culture medium, add 100μL high-sugar DMEM basal mediu...
Embodiment 2
[0062] Example 2 Screening Active Components with Anti-Adriamycin Hydrochloride Damaged Kidney Cells from Safflower Extract
[0063] Weigh 200g of safflower medicinal material, heat and reflux extraction with 50% ethanol twice, each time for 1h, combine the extracts, filter, concentrate and dry to obtain the A01 component. Fraction A01 was separated by macroporous resin, eluted with water, 20% ethanol, 40% ethanol, and 95% ethanol in sequence, and the eluents were collected and concentrated respectively to obtain B01, B02, B03, and B04 fractions. B02, B03 and B04 components were further separated by preparative liquid chromatography, and C01-C20, D01-D20 and E01-E20 components were prepared respectively. 53 safflower fractions (D15, D17, D19, D20, E05, E07, E11, E13, E16, E17, E19, E20 fractions were prepared too little to meet the screening requirements) were evaluated for renal cytoprotective activity. The specific process is:
[0064] An appropriate amount of components w...
Embodiment 3
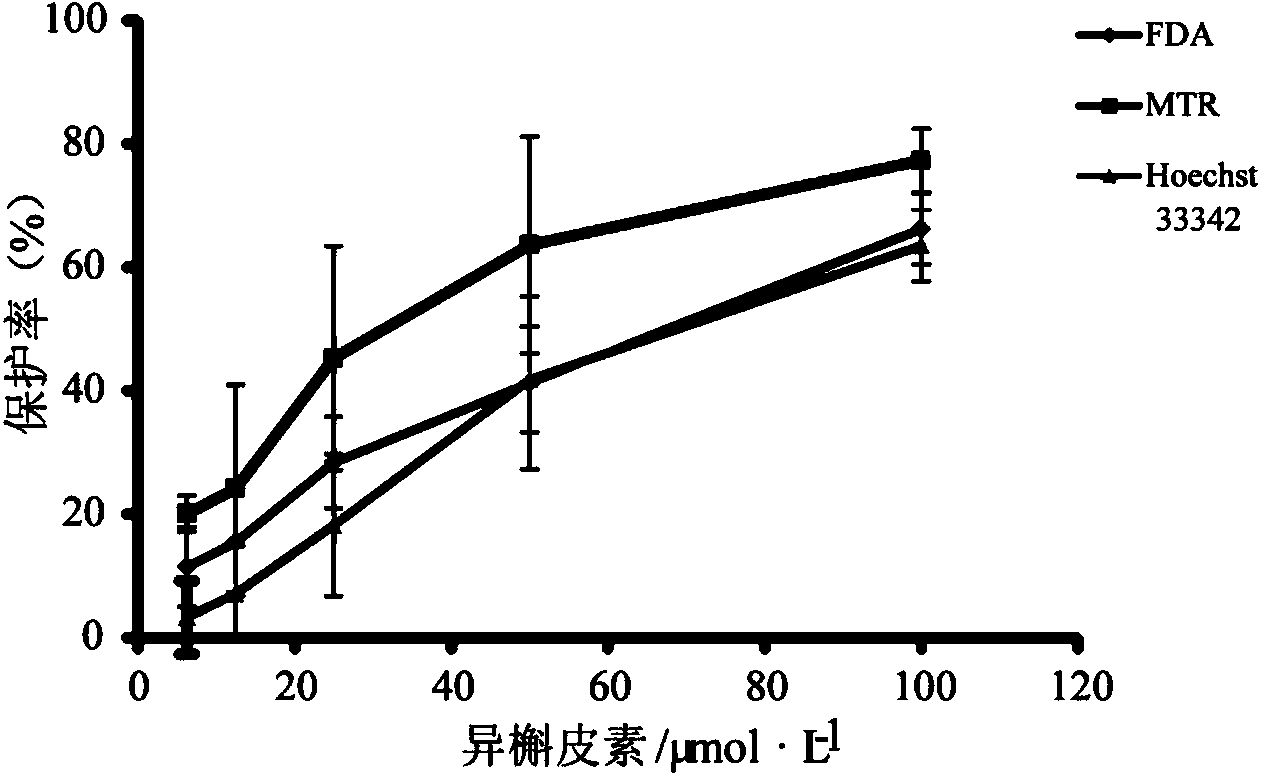
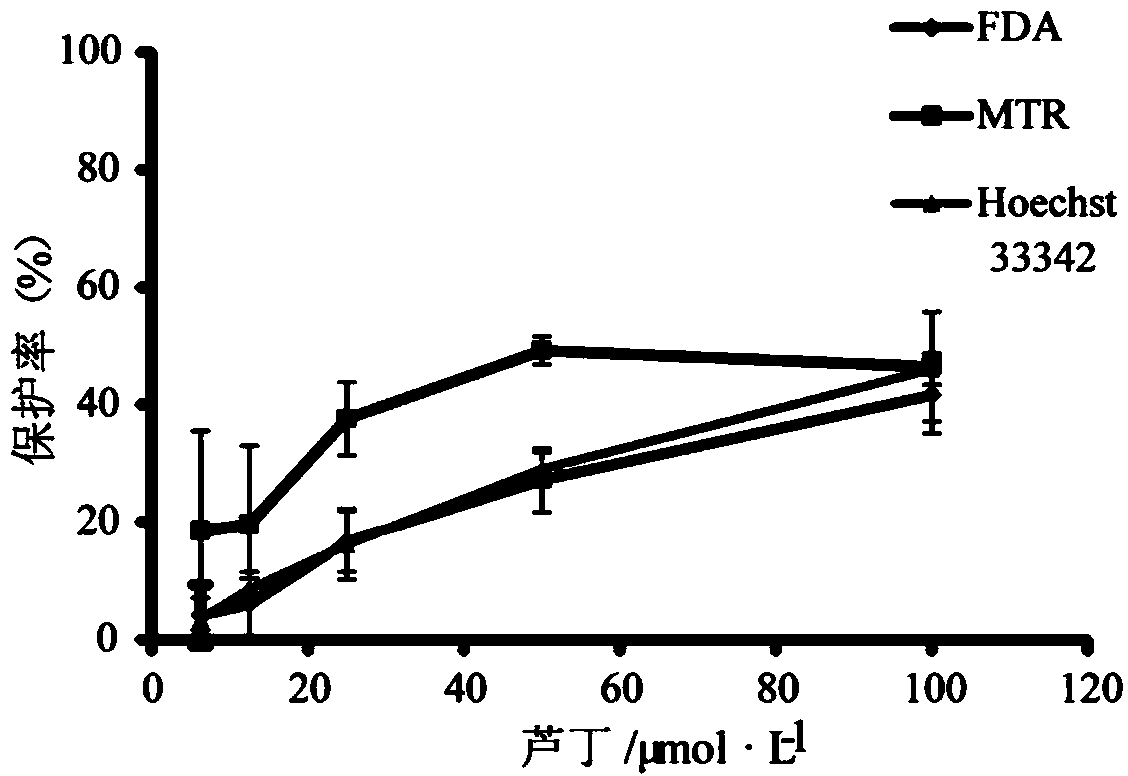
[0074] Example 3 Study on the activity of four active ingredients in safflower against doxorubicin hydrochloride-injured kidney cells
[0075] Select four active ingredients from Table 1: isoquercetin, rutin, kaempferol-3-O-rutinoside and hydroxysafflower yellow A, and dilute them into 5 concentrations (100, 50, 25, 12.5 and 6.25 μmol / L), using the method disclosed in the present invention (Example 2) to evaluate the protective effect of each compound on HK-2 cells damaged by doxorubicin hydrochloride, the evaluation results are shown in figure 2 , image 3 , Figure 4 , Figure 5 .
[0076] The experimental results show that the four compounds all have a certain degree of protective effect on HK-2 cells damaged by doxorubicin hydrochloride. Among them, the protective effect of hydroxysafflor yellow A is relatively weak, the protective effect of kaempferol-3-O-rutinoside and rutin is stronger, and the protective effect of isoquercetin is the strongest. Moreover, isoquerc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com