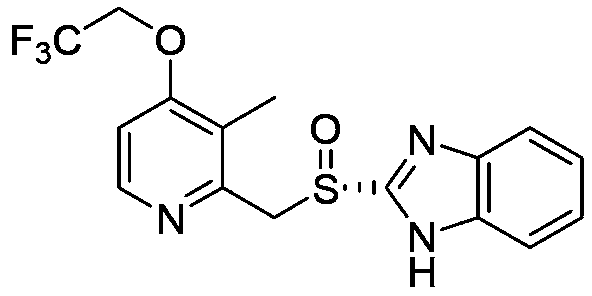
A kind of dexlansoprazole freeze-dried composition for injection and preparation method thereof
A technology of dexlansoprazole and composition, which is applied in the field of dexlansoprazole freeze-dried composition for injection and its preparation field, can solve the problem of short maintenance time of dexlansoprazole clarity, freeze-dried powder Problems such as poor resolubility and poor practicability of injections, to achieve the effects of easy preparation, simple preparation and rapid onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Each 1000 vials of lyophilized preparation contains the following components:
[0032]
[0033] Weigh each component according to the above prescription quantity, dissolve it in sterile water for injection with 80% of the prescription quantity, adjust the solution to clarification with an appropriate amount of sodium hydroxide solution, decolorize it with activated carbon, filter and decarbonize it with 1mol / L Adjust the pH value to 12.0 with sodium hydroxide, then add water to the full amount, and after fine filtration with a 0.22 μm microporous membrane, fill it in 1000 10mL vials under aseptic conditions, so that the main drug content is 30mg / bottle.
[0034]Freeze-drying: quickly cool down the vial filled with the composition liquid to -45°C, and keep it warm for about 120 minutes (to ensure that the product is frozen). Heat up to -20°C in about 360 minutes; keep warm until the water line disappears, and continue to keep warm until the product temperature is abov...
Embodiment 2
[0036] Each 1000 vials of lyophilized preparation contains the following components:
[0037]
[0038] The former and auxiliary materials of above-mentioned prescription are prepared into 1000 bottles of dexlansoprazole freeze-dried preparations for injection according to the method of embodiment 1 (when adjusting pH, 1mol / L potassium hydroxide is used to adjust to pH12.5). It is 15mg / support.
Embodiment 3
[0040] Each 1000 vials of lyophilized preparation contains the following components:
[0041] Dexlansoprazole 10g
[0042] Arginine 80g
[0043] Sorbitol 20g
[0044] The former and auxiliary materials of above-mentioned prescription are prepared into 1000 bottles of dexlansoprazole freeze-dried preparations for injection according to the method of embodiment 1 (when adjusting pH, use 2mol / L disodium hydrogen phosphate to adjust to pH11.5). The content is 10mg / support.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com