Preparation method and application of star polymer
A star-shaped polymer, polymer technology, applied in chemical instruments and methods, chemicals for biological control, disinfectants, etc., can solve problems that are rarely discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0064] In the first step, the following cationic amphiphilic linear copolymer L1 is prepared:
[0065]
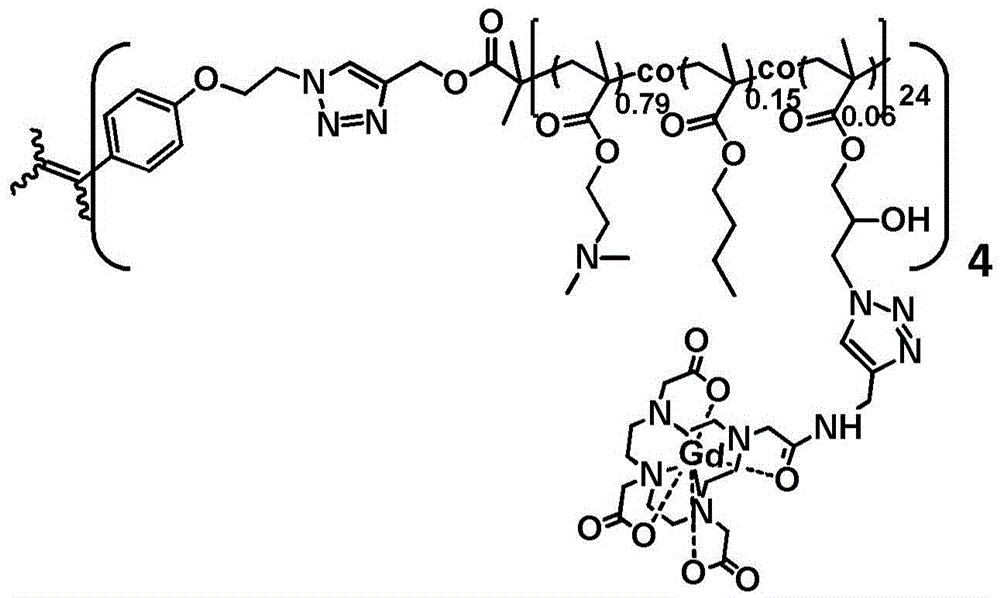
[0066] It is characterized in that: while the end group is an alkynyl group, it contains a positively charged tertiary amine monomer, a hydrophobic long alkyl chain monomer, and a glycidyl ester monomer that can be modified by sodium azide.
[0067] Preparation method: use polydentate nitrogen ligand and transition metal halide as catalyst, propynyl bromoisobutyrate as initiator, dimethylaminoethyl methacrylate, n-butyl methacrylate, shrink methacrylate Glyceride is a monomer, undergoing atom transfer radical random copolymerization. Proynyl bromoisobutyrate (25mg, 0.13mmol), dimethylaminoethyl methacrylate (1.63g, 10.4mmol), n-butyl methacrylate (0.08g, 0.56mmol), glycidyl methacrylate Esters (50mg, 0.35mmol), pentamethyldiethylenetriamine (41mg, 0.23mmol) were dissolved in anisole (2mL), fully degassed and equilibrated at 40°C for 10 minutes, then cuprous bromide (16m...
preparation example 2
[0077] In the first step, the following amphiphilic linear copolymer L2 is prepared:
[0078]
[0079] It is characterized in that: while the end group is an alkynyl group, it contains a positively charged tertiary amine monomer, a hydrophobic long alkyl chain monomer, and a glycidyl ester monomer that can be modified by sodium azide.
[0080] Preparation method: similar to the preparation method in Preparation Example 1, adjusting the feed ratio of monomers participating in atom transfer radical polymerization (ATRP). Dissolve propynyl bromoisobutyrate, dimethylaminoethyl methacrylate, n-butyl methacrylate, glycidyl methacrylate, and pentamethyldiethylenetriamine in anisole in turn. After degassing, equilibrate at 40°C for 10 minutes, then quickly add cuprous bromide. After 4 hours, the polymerization was terminated, the metal salt catalyst was removed, concentrated, and precipitated three times in diethyl ether. Obtain white solid product (0.54g, productive rate~40% aft...
preparation example 3
[0086] In the first step, the following linear copolymer L3 is prepared:
[0087]
[0088] It is characterized in that: while the end group is an alkynyl group, it contains a positively charged tertiary amine monomer and a glycidyl ester monomer that can be modified by sodium azide.
[0089] Preparation method: similar to the preparation method in Preparation Example 1, adjusting the feed ratio of monomers participating in atom transfer radical polymerization (ATRP). Propynyl bromoisobutyrate, dimethylaminoethyl methacrylate, glycidyl methacrylate, and pentamethyldiethylenetriamine were dissolved in anisole in turn for atom transfer radical polymerization (ATRP) . After 4 hours, the polymerization was terminated, and the metal salt catalyst was removed, followed by concentration and precipitation. Obtain white solid product (0.68g, productive rate~52% after vacuum drying; The GPC test result with tetrahydrofuran as mobile phase is: number average molecular weight M n =3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com