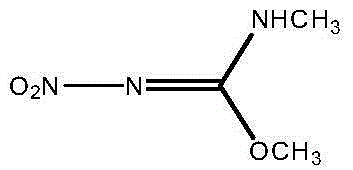
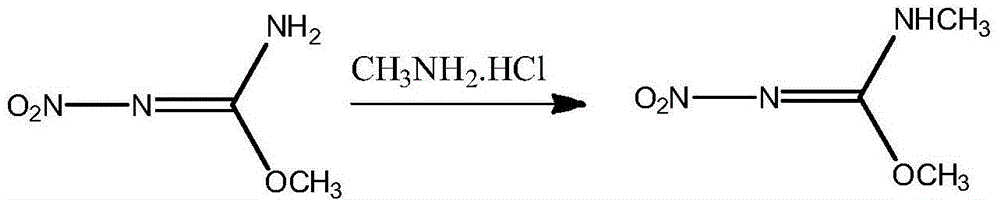
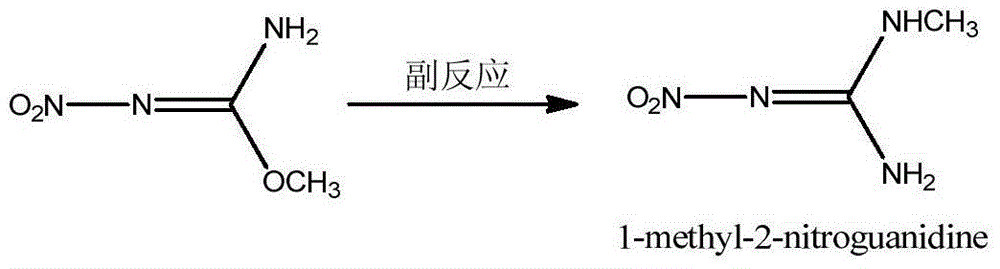
Preparation method of N,O-dimethyl-N'-nitroisourea
A technology of nitroisourea and dimethyl, which is applied in the field of preparation of N,O-dimethyl-N'-nitroisourea, can solve the problem of low reaction yield, achieve simple operation and suppress side reactions , the effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 250mL three-necked bottle equipped with mechanical stirring and a thermometer, add 24.3g (200mmol) of 98% O-methyl-N-nitroisourea, 13.5g (200mmol) of methylamine hydrochloride, 97.2ml Tap water and 19.0g (200mmol) potassium fluoride were reacted at 15°C for 2 hours. After the reaction was completed, the product was cooled to 0°C and filtered. The product was washed with 20mL of tap water at 5°C and dried to obtain 20.2g of a white product. The liquid chromatography quantification was 99.3 %, the yield is 75.4% based on O-methyl-N-nitroisourea.
Embodiment 2
[0018] In a 250mL three-necked flask equipped with mechanical stirring and a thermometer, add 24.3g (200mmol) of 98% O-methyl-N-nitroisourea, 27.0g (400mmol) of methylamine hydrochloride, 97.2ml Tap water and 57.0g (600mmol) potassium fluoride were reacted at 35°C for 2 hours. After the reaction was completed, cooled to 0°C and filtered, the product was washed with 20mL of tap water at 5°C, and dried to obtain 21.1g of a white product. The liquid chromatography quantification was 99.5 %, the yield is 78.9% based on O-methyl-N-nitroisourea.
Embodiment 3
[0020] In a 250mL three-necked flask equipped with a mechanical stirrer and a thermometer, add 24.3g (200mmol) of 98% O-methyl-N-nitroisourea, 20.3g (200mmol) of methylamine hydrochloride, 97.2ml Tap water and 38.0g (400mmol) potassium fluoride were reacted at 25°C for 5 hours. After the reaction was completed, cooled to 0°C and filtered, the product was washed with 20mL of tap water at 5°C, and dried to obtain 21.2g of a white product. The quantitative value of liquid chromatography was 99.2 %, the yield is 79.1% based on O-methyl-N-nitroisourea.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com