Pneumolysin (Ply) mutant and application thereof as mucosal immunoadjuvant
A technology for Streptococcus pneumoniae and mutants, which is applied in the field of medicine and biology, can solve the problems of high toxicity and cannot be applied to the human body, and achieves the effect of facilitating separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
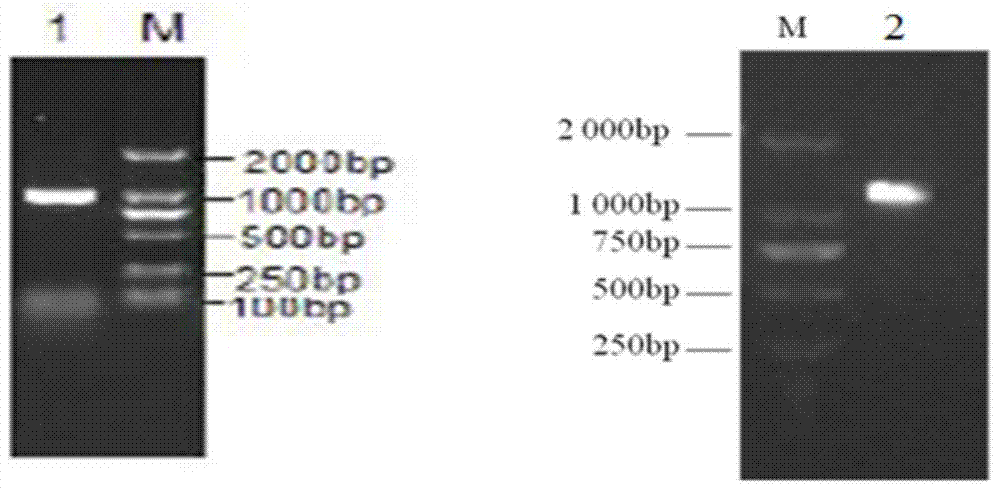
[0032] Construction of recombinant expression plasmids pET28a(+)-ΔPly, pET28a(+)-DnaJ, pET28a(+)-DnaJ-ΔPly and pET28a(+)-ΔPly-DnaJ expression vectors
[0033] (1) Materials:
[0034] Plasmid pET28a(+) was purchased from Novagen, Prime Star high-fidelity enzyme, dNTPs, Buffer, MgCl for PCR 2 Purchased from Dalian Bao Biological Technology Co., Ltd., PTC-200 PCR instrument is a product of Perkin Elmer, and RG-3000 is a product of Corbett Research.
[0035] (2) Design and synthesis of primers:
[0036] Using Streptococcus pneumoniae TIGR4 genomic DNA as a template, referring to its complete sequence (GeneBank number AE005672), primers were designed using Premier 5.0 and synthesized by Shanghai Sangong Company.
[0037] Construction reference of pET28a(+)-ΔPly recombinant plasmid (Lea-Ann S.Kirkham, Infect.Immun.2006,74(1):586.), construction reference of pET28a(+)-DnaJ recombinant plasmid (Mohd.Nadeem Khan , Vaccine 24 (2006) 6225–6231.)
[0038] DnaJ: upstream primer: 5'-GGA...
Embodiment 2
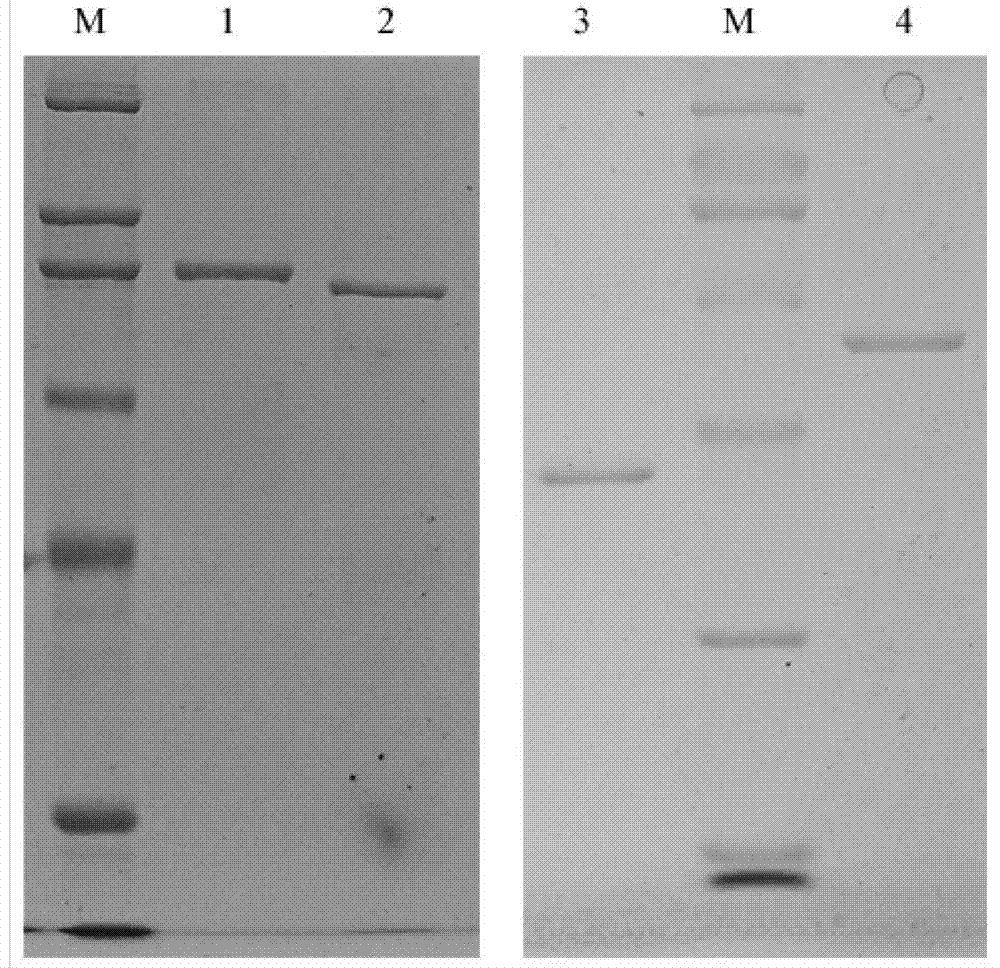
[0117] Expression, Identification and Purification of Prokaryotic Expression Plasmids pET28a(+)-ΔPly, pET28a(+)-DnaJ, pET28a(+)-DnaJ-ΔPly and pET28a(+)-ΔPly-DnaJ in Escherichia coli
[0118] (1) Transformation of recombinant plasmids pET28a(+)-ΔPly, pET28a(+)-DnaJ, pET28a(+)-DnaJ-ΔPly and pET28a(+)-ΔPly-DnaJ into the host strain BL21(DE3)
[0119] (2) IPTG induces the massive expression of ΔPly, DnaJ, DnaJ-ΔPly and ΔPly-DnaJ
[0120] (3) Purification of the recombinant protein: After the bacteria were broken by ultrasound, the supernatant of the broken bacteria was taken for purification; 4°C, 10000rpm×10min, the supernatant was filtered with a 0.45μm membrane filter, and the filtrate was collected for later use.
[0121] Affinity chromatography purification: aspirate 2ml of 50% Ni 2+ -NTA resin suspension in the chromatography column, equilibrate with 20ml sonication buffer; suck out the equilibrated Ni 2+ -NTA resin suspension and the above filtrate are fully mixed, ice-ba...
Embodiment 3
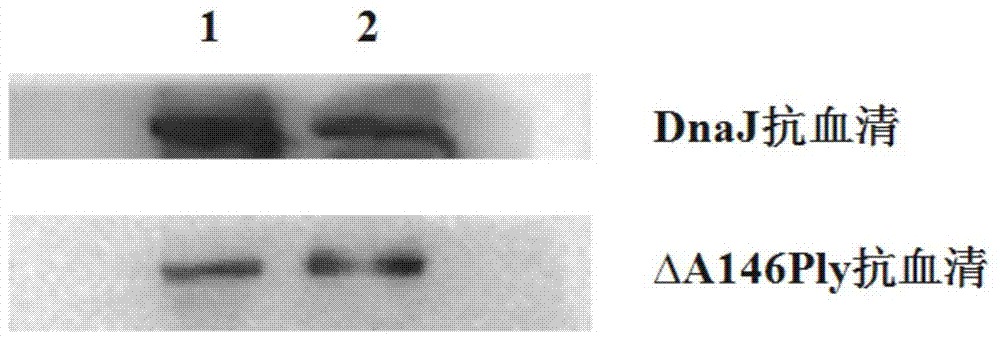
[0135] Evaluation of the effect of ΔPly as an adjuvant for mucosal immunity
[0136] (1) C57 mice were randomly divided into 6 groups, including single protein immunization group (1 group in total, DnaJ group), pairwise mixed immunization group (2 groups in total, DnaJ+ΔPly group and DnaJ+GST group respectively) ) and fusion protein immunization groups (group 2, DnaJ-ΔPly group and ΔPly-DnaJ group) and the positive control group of CT adjuvant (group 1, group CT+DnaJ).
[0137] (2) For the first immunization, use sterile PBS to adjust the recombinant protein concentration and immunize the mice in the experimental group by nasal drop, each 30ul, containing 8ugDnaJ and / or 10ugΔPly recombinant protein, 18ug of fusion protein, and 1ug of CT adjuvant;
[0138] (3) One week after the first immunization, the second immunization was carried out, the method and dose were the same as above, and the CT adjuvant was halved.
[0139] (4) Two weeks after the first immunization, the third i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com