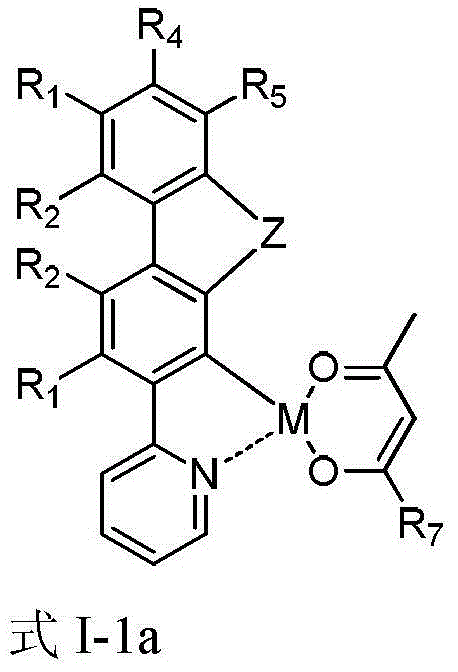
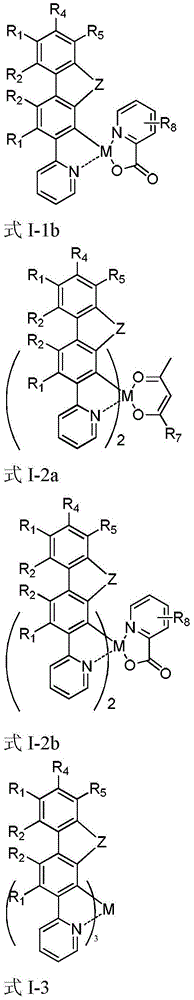
Series organic electrophosphorescent material
A compound and selected technology, applied in the fields of luminescent materials, organic chemistry, circuits, etc., can solve the problems of small luminous contribution and difficult to improve luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The preparation of embodiment 1 compound PHIR-AC-005
[0090]
[0091] Step 1: Preparation of 6,6′-difluorobiphenyl-2,2′-dicarboxylic acid
[0092]
[0093] Dissolve 75g of copper sulfate pentahydrate in 300ml of water, add 230ml of 25% ammonia water under stirring, and cool down to 0°C with an ice-salt bath. An aqueous solution prepared by 20.8 g of hydroxylamine hydrochloride and 12.8 g of sodium hydroxide was slowly added dropwise into the above cold copper solution, and kept below 0° C. for later use.
[0094] Mix 31g of 2-amino-3-fluorobenzoic acid and 85ml of concentrated hydrochloric acid, add 300ml of water and 60ml of acetonitrile, and cool down to below 0°C with an ice-salt bath, slowly drop the solution of 15.9g of sodium nitrite dissolved in 120ml of water Add to the above solution, stir and react at below 5°C for 1 hour, slowly add this clear diazonium solution dropwise into the reserved copper solution, and keep the temperature below 10°C during thi...
Embodiment 2
[0127] The preparation of embodiment 2 compound PHIR-AC-006
[0128]
[0129] The first step: preparation of compound G-2
[0130]
[0131] 1.0g of compound 2-(4,5-bistrifluoromethyl-9,10-dihydrophenanthrene-2-yl)pyridine (refer to Example 1, the synthesis of the first to eight steps) and 427mg of IrCl 3 ·3H 2 O was dispersed in 30ml of ethylene glycol ether and 10ml of water, under the protection of nitrogen, the temperature was raised to reflux for 24 hours, cooled to room temperature, filtered, the filter cake was washed with water, and dried in vacuo to obtain 520mg of compound G-2 as a yellow solid.
[0132] The second step: the preparation of compound PHIR-AC-006
[0133]
[0134] 500mg of compound G-2, 62mg of acetylacetone and 262mg of anhydrous sodium carbonate were dispersed in 40ml of ethylene glycol ether, under the protection of nitrogen, the temperature was raised to reflux for 24 hours, cooled to room temperature, and the reaction solution was poured ...
Embodiment 3
[0140] The preparation of embodiment 3 compound PHIR-AC-001
[0141]
[0142] The first step: the preparation of 2-(4,5-difluorophenanthrene-2-yl)pyridine
[0143]
[0144] 1.5g of 2-(4,5-difluoro-9,10-dihydrophenanthrene-2-yl)pyridine prepared in the eighth step of Example 1 was dissolved in 100ml of benzene, 3.5g of DDQ was added, and heated to reflux for reaction After 3 days, it was cooled to room temperature, concentrated to dryness under reduced pressure, and the residue was separated and purified by a neutral alumina column to obtain 1.4 g of 2-(4,5-difluorophenanthrene-2-yl)pyridine as a white solid.
[0145] The second step: the preparation of compound G-2
[0146]
[0147] Referring to the ninth step of Example 1, replace 2-(4,5-difluoro-9,10-dihydrophenanthrene-2-yl)pyridine with 2-(4,5-difluorophenanthrene-2-yl ) pyridine to obtain compound G-2, a yellow solid.
[0148] The third step: preparation of compound PHIR-AC-001
[0149]
[0150] Referring ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com