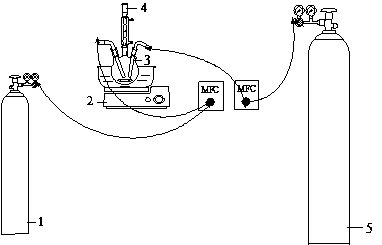
Method for performing catalytic oxidation on hydrogen sulfide through 1-butyl-3-methylimidazole ethylene diamine tetraacetic acid (EDTA) iron
A technology of alkylimidazole EDTA iron and alkylimidazole EDTA iron liquid, which is applied in the preparation/purification of sulfur, can solve the problems of easy scaling and reduce the purity of sulfur products, and achieve low Effects of surface tension, rapid and efficient oxidation removal, and avoidance of by-products such as thiosulfate and sulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 4.8g NaOH dissolved in 100ml deionized water, 10.8g FeCl 3 ·H 2 O is dissolved in an appropriate amount of deionized water (the amount of water is measured as FeCl 3 ·H 2 O to form a clear and transparent solution) After forming a clear and transparent solution, add it to the NaOH aqueous solution, and stir slowly until a suspension is formed. Then pour the suspension into a 1000ml beaker, add tap water to 800ml, and wash 3-4 times. Filter under reduced pressure to obtain Fe(OH) 3 solid. After mixing with 10.52g of ethylenediaminetetraacetic acid and 74ml of deionized water, heat and boil until a brown transparent solution is formed, and cool to room temperature to obtain a solution of Fe-EDTA.
Embodiment 2
[0051] In the Fe-EDTA solution prepared in Example 1, add 1-butyl-3-methylimidazole hydroxide ([Bmim]OH) and stir for 10 minutes, and after rotary evaporation of water at 50°C, brown [Bmim] was obtained [Fe(EDTA)] viscous solution, the pH of the solution is 5.58, the water content is 32.5%, the EDTA content is 29.2%, and the Fe concentration is 5.67%.
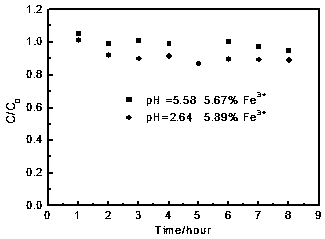
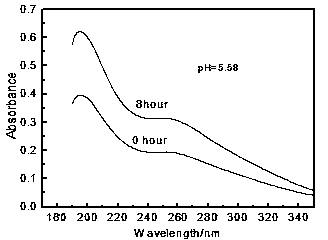
[0052] Take 55mL of [Bmim][Fe(EDTA)] aqueous solution and inject it into the desulfurization reactor, at 30°C, control 99.9%O 2 The flow rate is 25mL / min, 99.9%H 2 The S flow rate is 20mL / min, and the catalytic-oxidation in the desulfurization absorption liquid is measured every hour. After 8 hours, its concentration still reaches 95% of the initial concentration (EDTA) complexing agent content. The ratio of the concentration after EDTA catalyzed-oxidation reaction to the initial concentration varies with time as figure 2 shown. Depend on figure 2 It can be seen that when the [Bmim][Fe(EDTA)] solution with a pH of 5.58 an...
Embodiment 3
[0056] In the Fe-EDTA solution prepared by the same method as in Example 1, add 1-butyl-3-methylimidazole hydroxide ([Bmim]OH) and stir for 10 minutes. After rotary evaporation of water at 50°C, brown The [Bmim][Fe(EDTA)] viscous solution has a pH of 2.64, a water content of 43.5%, a Fe concentration of 5.89%, and an EDTA content of 30.3%.
[0057] Take 47.5 mL of [Bmim][Fe(EDTA)] aqueous solution and inject it into the desulfurization reactor, at 30°C, control 99.9% O 2 The flow rate is 25mL / min, 99.9%H 2 The flow rate of S is 20mL / min, and the content of ethylenediaminetetraacetic acid (EDTA) complexing agent in the desulfurization absorption liquid is measured every hour. The ratio of the concentration after EDTA catalyzed-oxidation reaction to the initial concentration varies with time as figure 2 shown. Depend on figure 2 It can be seen that the [Bmim][Fe(EDTA)] solution with a pH of 2.64 catalyzed-oxidized hydrogen sulfide for 8 hours, and its concentration reached...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com