Method for measuring residual solvent in bulk drug mifepristone
A technology for mifepristone and residual solvent is applied in the field of determination of residual solvent in raw material mifepristone, which can solve problems such as hidden dangers of drug safety, and achieve the effects of high sensitivity, quick and simple operation, and improved safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
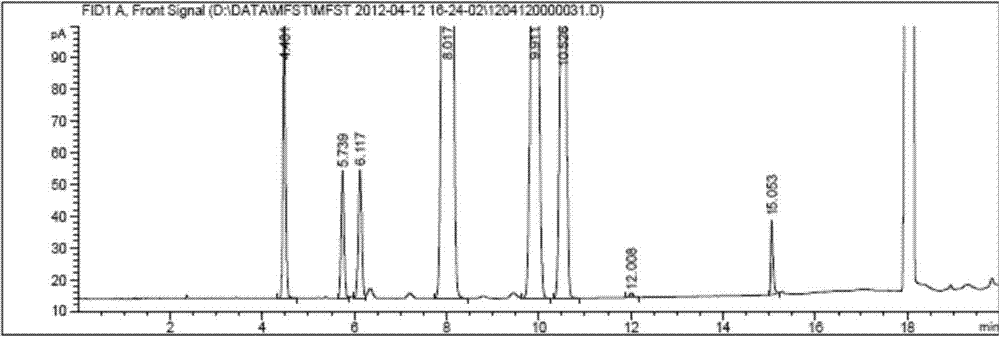
[0050] The mensuration of residual solvent in the bulk drug mifepristone of embodiment 1
[0051] (1) Analytical method: headspace gas chromatography.
[0052] Chromatography system:
[0053] Chromatographic column: Agilent DB-624 elastic quartz capillary column (30m×0.53mm×3μm), FID detector.
[0054] Chromatographic parameters:
[0055] Chromatographic separation parameters: carrier gas: nitrogen; carrier gas flow rate: 3.0mL / min; split ratio: 10:1;
[0056] Detector temperature: 250°C; Injection port temperature: 200°C;
[0057] Column temperature: the initial temperature is 45°C, keep for 10 minutes, increase the temperature to 150°C at a rate of 15°C / min, and keep for 3 minutes.
[0058] Headspace detection parameters: heating box: 105°C; quantitative loop: 115°C; transfer line: 125°C;
[0059] GC cycle time: 25min; injection time: 1min;
[0060] Sample balance time: 20min; pressure balance time: 0.2min.
[0061] (2) Solution preparation:
[0062] 1. Reference sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com