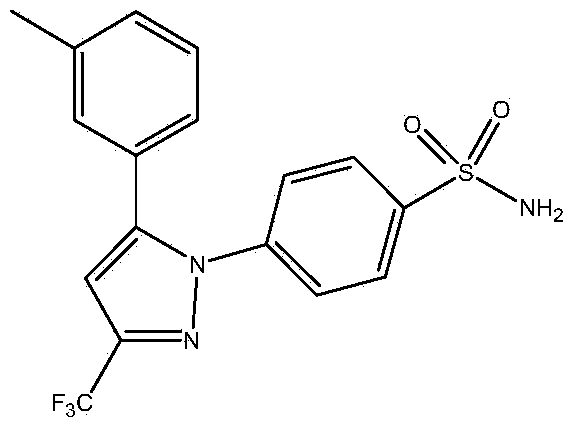
Synthetic method of celecoxib
A synthetic method, celecoxib technology, applied in the field of medicinal chemistry, can solve the problems of multiple recrystallization times, complex solvent system, and difficult solvent recovery, and achieve the effects of high purity, simple operation, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment one: a kind of synthetic method of celecoxib
[0031] 1L four-necked reaction flask, equipped with mechanical stirring, water bath. Under nitrogen protection, 12.8g of sodium methylate and 200ml of industrial toluene were first added thereto. Control the internal temperature not to exceed 40°C, and add 30 g of ethyl trifluoroacetate dropwise. After dropping, cool to about 30°C, add 25g of p-methylacetophenone dropwise, no heat release at this time. Stir at room temperature for 1 hour, then gradually raise the temperature to 110°C and react for about 5 hours. The central control checks that the content of p-methylacetophenone is less than 1%, and the reaction is stopped. After the reaction, add 100ml of 3N hydrochloric acid solution to the reaction solution to adjust the pH to 2, stir for 10 minutes until the organic layer is clear, and let stand to separate layers; the aqueous layer is extracted with 50ml of toluene, and the combined organic layers are wash...
Embodiment 2
[0035] Embodiment two: a kind of synthetic method of celecoxib
[0036] 1L four-necked reaction flask, equipped with mechanical stirring, water bath. Under nitrogen protection, 12.5 g of sodium methoxide and 200 ml of industrial toluene were added. Control the internal temperature not to exceed 40°C, and add 30 g of ethyl trifluoroacetate dropwise. After dropping, cool to about 30°C, add 25g of p-methylacetophenone dropwise, and stir at room temperature for 1 hour after adding. Gradually raise the temperature to 110°C and react for about 3 hours. The central control checks that the content of p-methylacetophenone is less than 1%, and the reaction is stopped. After the reaction, add 100ml of 3N hydrochloric acid solution to the reaction solution to adjust the pH to 2, stir for 10 minutes until the organic layer is clear, and let stand to separate layers; the aqueous layer is extracted with 50ml of toluene, and the combined organic layers are washed once with 60ml of water.
...
Embodiment 3
[0040] Embodiment three: a kind of synthetic method of celecoxib
[0041] 1L four-necked reaction flask, equipped with mechanical stirring, water bath. Under nitrogen protection, 12.5 g of sodium methoxide and 200 ml of industrial toluene were added. Control the internal temperature not to exceed 40°C, and add 31 g of ethyl trifluoroacetate dropwise. After dropping, cool to about 30°C, add 25g of p-methylacetophenone dropwise, and stir at room temperature for 1 hour after adding. Gradually raise the temperature to 110°C and react for about 3 hours. The central control checks that the content of p-methylacetophenone is less than 1%, and the reaction is stopped. After the reaction, add 100ml of 3N hydrochloric acid solution to the reaction solution to adjust the pH to 2, stir for 10 minutes until the organic layer is clear, and let stand to separate layers; the aqueous layer is extracted with 50ml of toluene, and the combined organic layers are washed once with 60ml of water. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com