Method for catalytically oxidizing alkane and cycloparaffin by use of conjugated polymetalloporphyrin
A technology for polymerizing metals and metalloporphyrins, applied in chemical instruments and methods, catalytic reactions, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
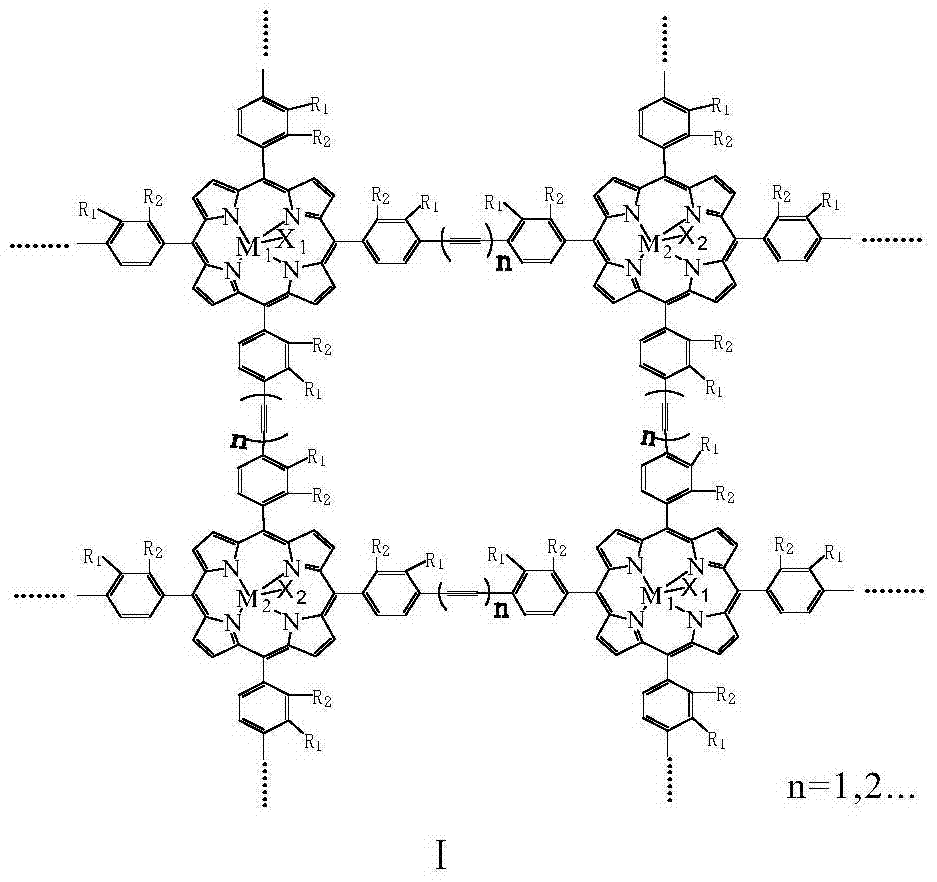
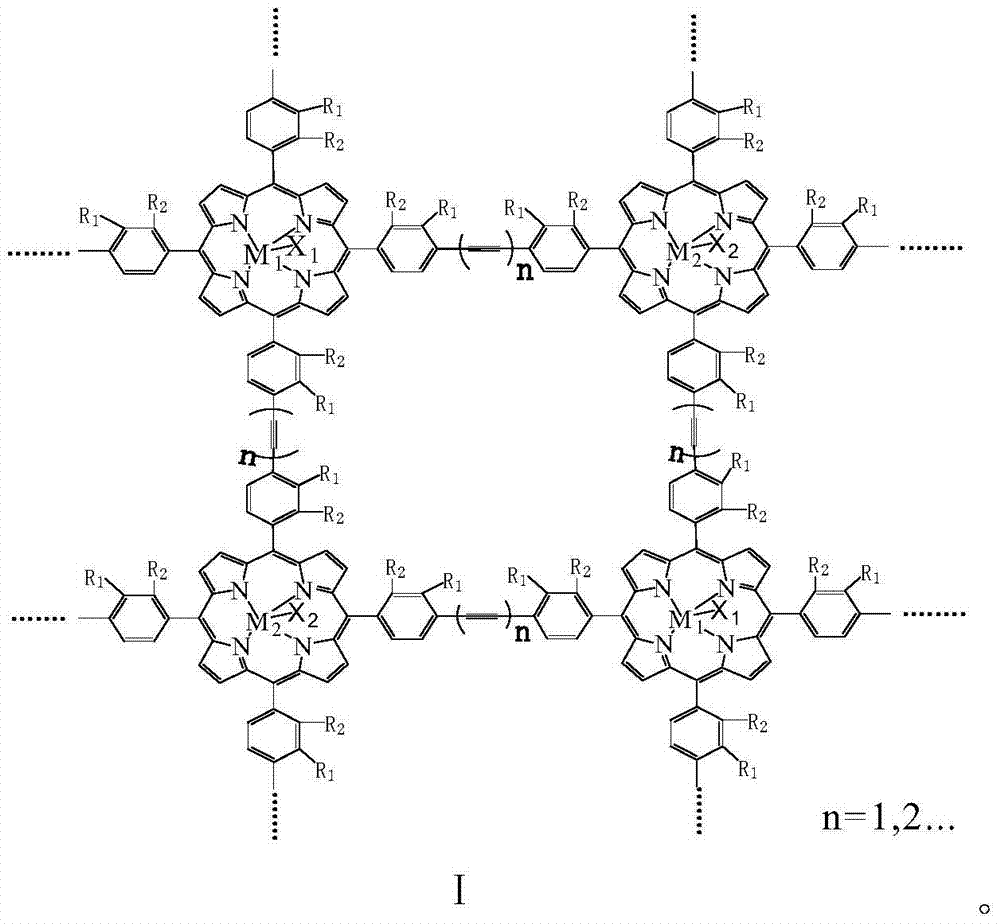
[0013] With 20mg of the metalloporphyrin polymer with structural formula I structure, R 1 =R 2 = H, M 1 = M 2 =Mn,X 1 =X 2 =Cl, n=1, molecular weight is 115000, specific surface area is 860m 2 / g, was added to 500ml cyclohexane, and 8atm air was introduced. The reactant was stirred at 125° C. for 2 hours, the conversion rate of cyclohexane was 18.1%, and the total yield of cyclohexanol and cyclohexanone in the reaction product was 92%.
Embodiment 2
[0015] With 20mg of the metalloporphyrin polymer with structural formula I structure, R 1 = H, R 2 =CH 3 ,M 1 = M 2 =Fe,X 1 =X 2 =Cl, n=2, molecular weight is 53000, specific surface area is 750m 2 / g, was added to 500ml cyclohexane, and 8atm air was introduced. The reactant was stirred at 100° C. for 4 hours, the conversion rate of cyclohexane was 8.6%, and the total yield of cyclohexanol and cyclohexanone in the reaction product was 94%.
Embodiment 3
[0017] With 20mg of the metalloporphyrin polymer with structural formula I structure, R 1 =R 2 =Cl,M 1 =Mn,M 2 =Fe,X 1 =X 2 =Cl, n=1, molecular weight is 172000, specific surface area is 970m 2 / g, add 500ml of cyclohexane, and introduce l0atm air. The reactant was stirred at 130° C. for 2 hours, the conversion rate of cyclohexane was 21.4%, and the total yield of cyclohexanol and cyclohexanone in the reaction product was 91%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com