Tumor-targeting polypeptide-doxorubicin derivative and its preparation method and application
A technology of tumor targeting and doxorubicin, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations. It can solve the problems of high transferrin concentration, increase drug concentration, and have simple and easy preparation methods and small steric hindrance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
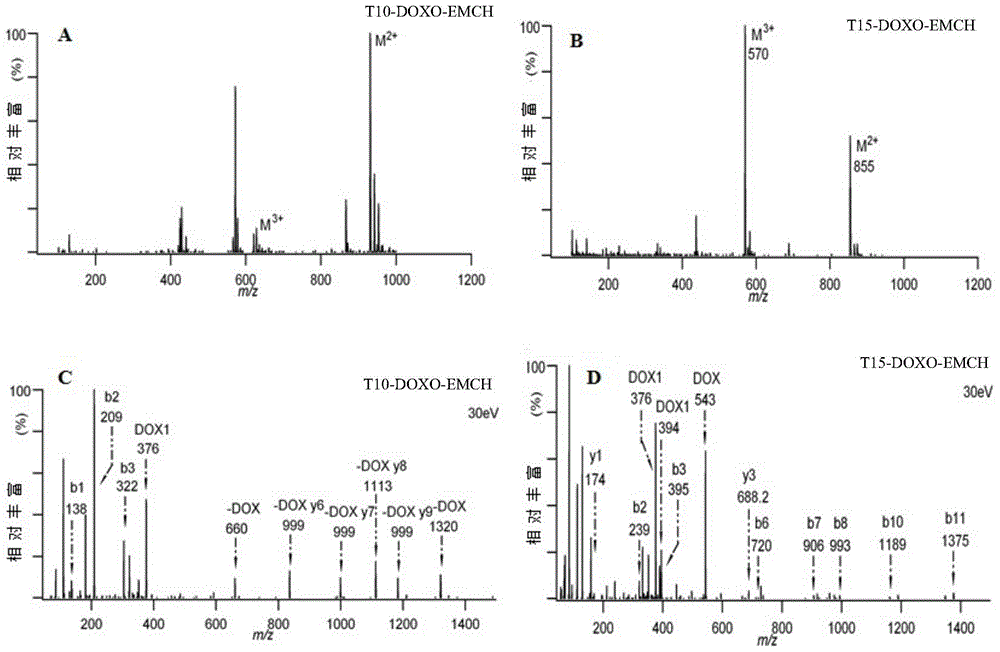
[0035] Synthesis and purification of embodiment 1 polypeptide-doxorubicin complex
[0036] (1) Preparation of T10-DOXO-EMCH and T15-DOXO-EMCH
[0037] Weigh 0.8 mg of T10 peptide, dissolve it in 600 μL of pH7.8-8.2 phosphate buffer, then weigh 1.13 mg of DOXO-EMCH, and dissolve it in 600 μL of N, N'-dimethylformamide aqueous solution, where N , the volume ratio of N'-dimethylformamide to water is 1:1, drop into the polypeptide solution at a rate of 25 μL / min, react at room temperature for 1.5 hours, and generate T10-DOXO-EMCH, transfer the solution to a centrifuge tube, Centrifuge at 8000×g for 5 min to remove unreacted substances to obtain a crude product.
[0038] The structure of doxorubicin prodrug (6-maleimidocaproyl) hydrazone derivative—DOXO-EMCH is as follows:
[0039]
[0040]Weigh 1.23mg of T15 peptide, dissolve in 600μL pH7.8~8.2 phosphate buffer, then weigh 1.13mg of DOXO-EMCH, dissolve in 600μL N,N'-dimethylformamide aqueous solution, where N , the volume ra...
Embodiment 2
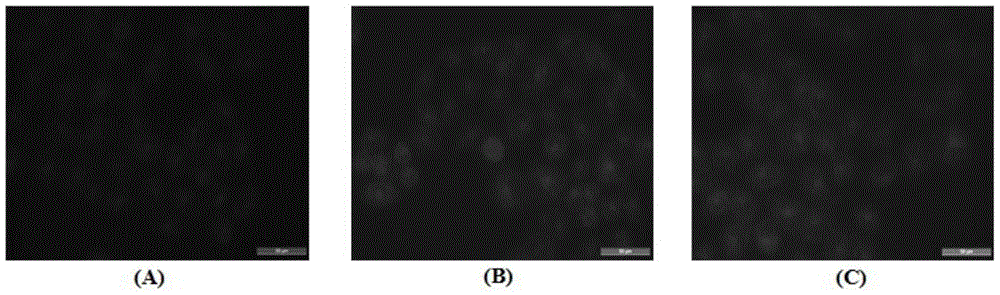
[0051] Example 2 Intracellular Adriamycin Accumulation Experiment
[0052] MCF-7 / ADR cells at 1×10 5 The density per well was inoculated in a 6-well culture plate, and after incubation at 37°C and 5% carbon dioxide for 24 hours, DOX and its derivatives T10-DOXO-EMCH and T15-DOXO-EMCH were added with a final concentration of 1.5 μM. The effect is 12h. Discard the drug-containing culture medium, wash the cells 3 times with cold PBS, and observe the treated cells in each group under a fluorescent microscope. The results are shown in figure 2 . After MCF-7 / ADR cells were treated with different drugs, the fluorescence intensity in the T10-DOXO-EMCH and T15-DOXO-EMCH groups was significantly higher than that in the DOX group.
Embodiment 3
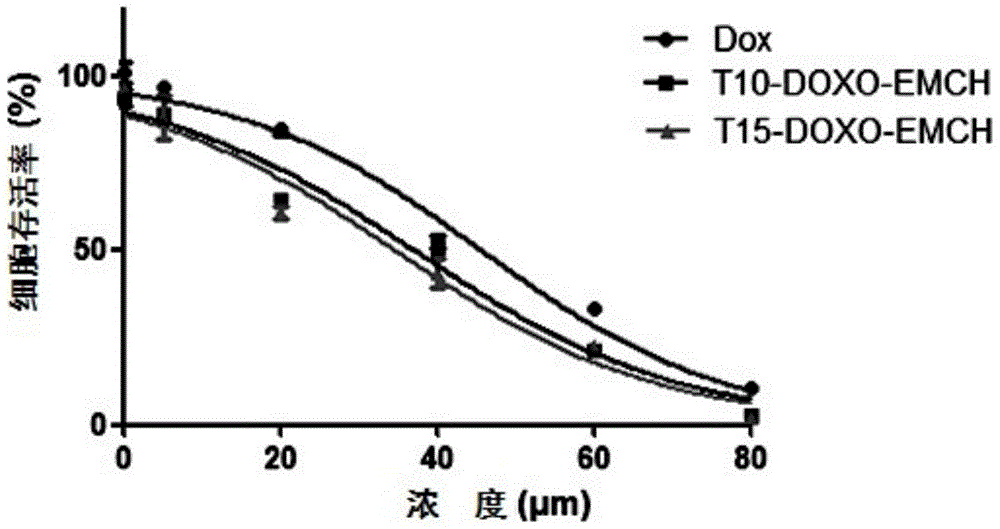
[0053] Embodiment 3MTT method measures cell viability test
[0054] Digest MCF-7 / ADR cells in good condition and dilute to 2×10 with culture medium 4 cells / mL cell density, after blowing evenly, add 200 μL of cell suspension to each well of a 96-well plate, and incubate at 37°C and 5% carbon dioxide for 24 hours to make it adhere to the wall. Replace with 200 μL DOX, T10-DOXO-EMCH and T15-DOXO-EMCH drug-containing culture medium, set 5 parallel wells for each concentration, and set a blank control group at the same time, place them in an incubator for 72 hours, and then add MTT solution (5 mg / mL) was 20 μL, continued to incubate for 4 h, the culture medium was sucked off, DMSO 200 μL was added, and the microplate shaker was shaken for 10 min at room temperature, and the absorbance value of each well was measured at 490 nm in an enzyme-linked immunosorbent assay instrument. Taking the control cells as 100%, the survival rate of the cells in the administration group relative to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com