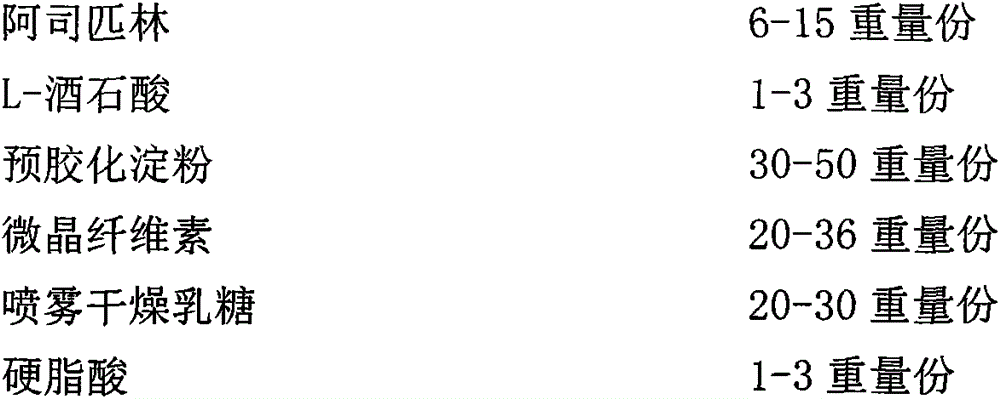Sustained-release tablet for treating cardiovascular diseases and preparation method thereof
A sustained-release tablet and cardiovascular technology, which is applied in the field of sustained-release tablets for the treatment of cardiovascular diseases and its preparation, and can solve problems such as the difficulty in meeting the quality control standards for free salicylic acid in aspirin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Embodiment 1: Aspirin dipyridamole sustained release tablet
[0163] Aspirin tablets:
[0164]
[0165] Isolation coat:
[0166] Opadry 80W 620004Yellow 3kg
[0167] 20% ethanol solution 28kg
[0168] Dipyridamole mixed granules:
[0169]
[0170] Protective Coating:
[0171] Opadry 295K 620010Yellow 8kg
[0172] 80% ethanol solution 126kg
[0173] Aspirin was crushed through a 30-mesh sieve, L-tartaric acid was crushed through a 80-mesh sieve, pregelatinized starch and spray-dried lactose were passed through a 18-mesh sieve, and stearic acid was passed through a 20-mesh sieve for later use; aspirin and L-tartaric acid were weighed according to the prescription amount. Mix tartaric acid, then add pregelatinized starch and mix, then add spray-dried lactose and mix, then add microcrystalline cellulose PH101 and mix, finally add stearic acid, mix evenly, and use Φ=9.5mm shallow concave punch to compress tablets; Dai 80W 620004Yellow barrier layer coating incre...
Embodiment 2
[0174] Embodiment 2: aspirin dipyridamole sustained release tablet
[0175] Aspirin tablets:
[0176]
[0177] Isolation coat:
[0178] Opadry 80W 620004Yellow 4kg
[0179] 10% ethanol solution 35kg
[0180] Dipyridamole mixed granules:
[0181]
[0182] Protective Coating:
[0183] Opadry 295K 620010Yellow 10kg
[0184] 95% ethanol solution 150kg
[0185] Grind aspirin through a 40-mesh sieve, L-tartaric acid through a 70-mesh sieve, pregelatinized starch and spray-dried lactose through a 10-mesh sieve, and stearic acid through a 30-mesh sieve for later use; weigh aspirin and L- Mix tartaric acid, then add pregelatinized starch and mix, then add spray-dried lactose and mix, then add microcrystalline cellulose PH101 and mix, finally add stearic acid, mix evenly, and use Φ=9.5mm shallow concave punch to compress tablets; Dai 80W 620004Yellow barrier layer coating increased to 3% in weight, used as aspirin barrier layer coating tablet core; dipyridamole, hypromellos...
Embodiment 3
[0186] Embodiment 3: aspirin dipyridamole sustained release tablet
[0187] Aspirin tablets:
[0188]
[0189] Isolation coat:
[0190] Opadry 80W 620004Yellow 2kg
[0191] 30% ethanol solution 20kg
[0192] Dipyridamole mixed granules:
[0193]
[0194] Protective Coating:
[0195] Opadry 295K 620010Yellow 6kg
[0196] 60% ethanol solution 100kg
[0197]Grind aspirin through a 20-mesh sieve, L-tartaric acid through a 90-mesh sieve, pregelatinized starch and spray-dried lactose through a 20-mesh sieve, stearic acid through a 10-mesh sieve, and set aside; weigh aspirin and L- Mix tartaric acid, then add pregelatinized starch and mix, then add spray-dried lactose and mix, then add microcrystalline cellulose PH101 and mix, finally add stearic acid, mix evenly, and use Φ=9.5mm shallow concave punch to compress tablets; Dai 80W 620004Yellow barrier layer coating increased to 2% in weight, used as aspirin barrier layer coating tablet core; dipyridamole, hypromellose K4M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com