A thermophilic alkaline recombinant manganese-containing catalase and its Pichia pastoris expression vector and engineering bacteria
A technology of manganese catalase and expression vector, which is applied in the field of thermophilic alkaline recombinant manganese-containing catalase and its expression vector of Pichia pastoris and engineering bacteria to achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The construction of embodiment 1 manganese catalase MnCAT yeast expression genetic engineering bacteria
[0045] 1.1 MnCAT gene designed based on Pichia pastoris codon usage preference optimization
[0046] According to the preference of codon usage in Pichia pastoris ( http: / / gcua.schoedl.de / seqoverall_v2.html ) optimization design, the resulting codon-optimized MnCAT gene has a nucleotide sequence as shown in SEQ ID No: 1.
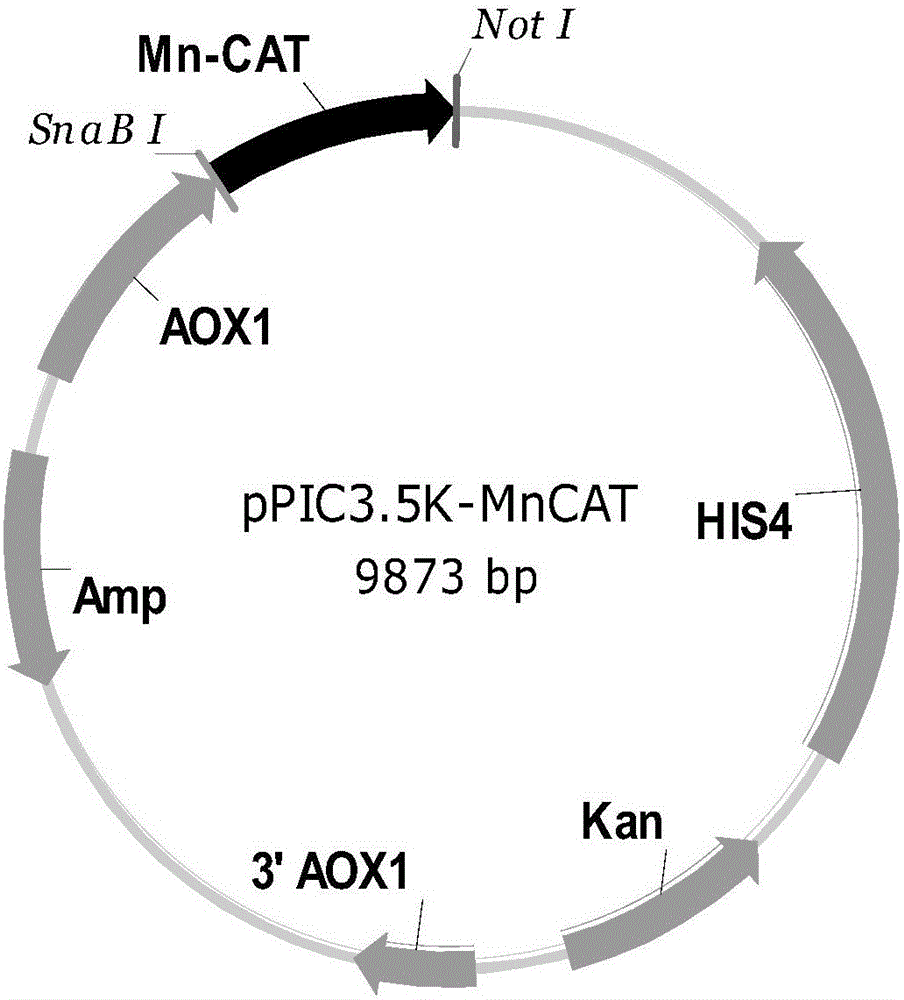
[0047] 1.2 Construction of MnCAT Yeast Expression Vector pPIC3.5K-MnCAT
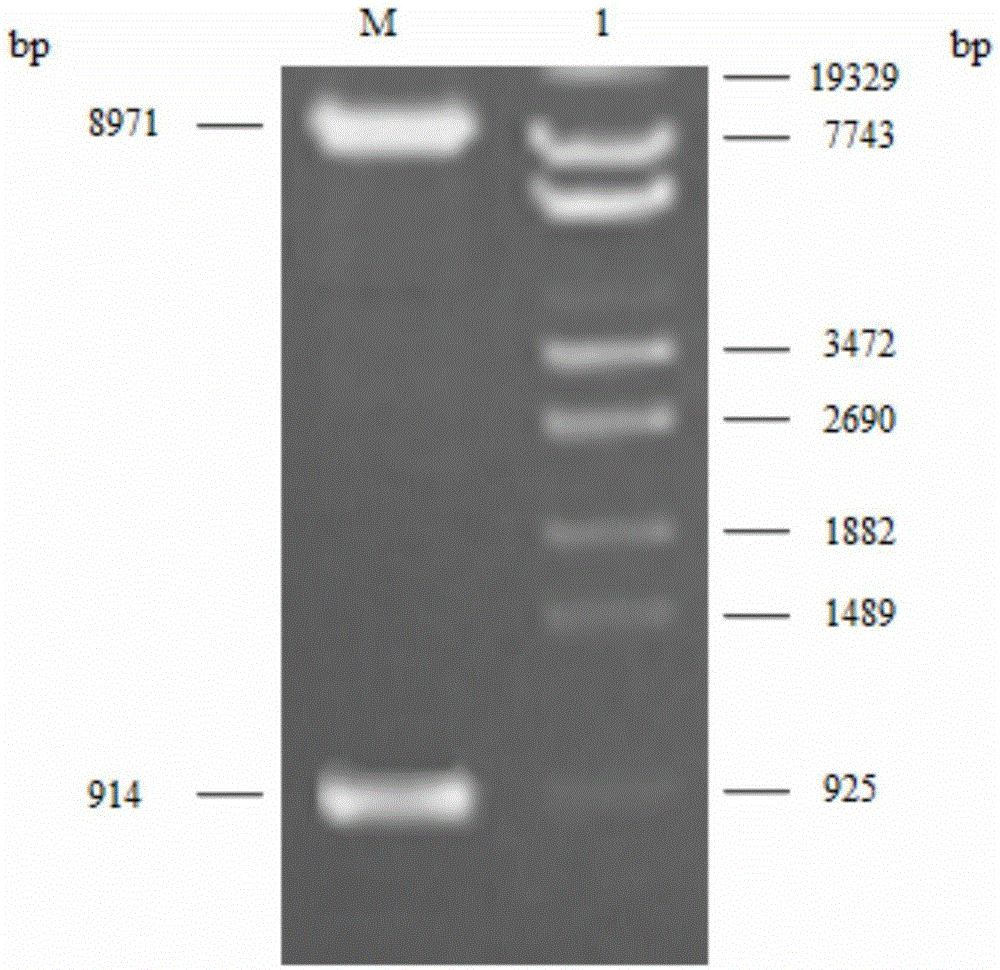
[0048] At the 5'-end and 3'-end of the MnCAT gene, the restriction endonuclease SnaBI and NotI restriction endonuclease sites that are not available in the gene but are available in the multiple cloning site of the vector pPIC3.5K were designed by Shanghai Sangon Biotechnology Directly synthesized by Engineering Technology Service Co., Ltd., the MnCAT gene fragment was digested by SnaBI and NotI, and then T4-ligated with the expression vector pPIC3.5K after the same digest...
Embodiment 2
[0053] Shake flask fermentation culture of embodiment 2 recombinant bacteria KM71 / pPIC3.5K-MnCAT
[0054] 2.1 Shake flask seed culture
[0055] Inoculate the recombinant strain KM71 / pPIC3.5K-MnCAT into 50mL of LYPD medium, and culture at 30°C and 200rpm / min for 19-22h;
[0056] 2.2 Shake flask fermentation culture:
[0057] Transfer the seed culture solution to 50mL BMGY medium according to the inoculum amount of 10%, culture at 30°C, 200r / min for 19-22h; collect the bacteria by centrifugation at 4°C, 6000r / min, and use 25mL containing the final concentration of 1mmol / LMnCl 2 Resuspended bacteria in BMMY medium, cultivated at 30°C and 200rpm / min, added 100% methanol to the medium every 20h to a final concentration of 0.25% (V / V) to induce expression of Mn-CAT, and cultured for 120h , collected the bacteria by centrifugation, broken the cells, and analyzed the supernatant by SDS-PAGE. It was found that there was an obvious protein band at 33Kda, which was consistent with the ...
Embodiment 3
[0062] 5L fermenter culture of embodiment 3 recombinant bacteria KM71 / pPIC3.5K-MnCAT
[0063] 3.1 Seed culture in fermenter
[0064] The recombinant strain KM71 / pPIC3.5K-MnCAT was inoculated into 50mL seed medium, and cultured at 30℃, 200rpm / min for 19-22h.
[0065] The components of the fermenter seed medium are: peptone 15g / L, yeast extract 15g / L, glycerol 10g / L, biotin 4×10-4g / L, potassium phosphate buffer 0.1mol / L (pH6.0).
[0066] 3.2 Fermentation tank fermentation
[0067] The seed culture solution was transferred to the BSM medium of the fermenter with a liquid content of 2L according to the inoculum amount of 10%. -20%; when the glycerol in the initial medium is exhausted, start to feed the feed medium, and control the final concentration of glycerol in the culture medium to be less than 10g / l; when the recombinant bacteria grow to OD 600 When the temperature is 100-120, start to add methanol continuously, control the final volume concentration of methanol in the cult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com