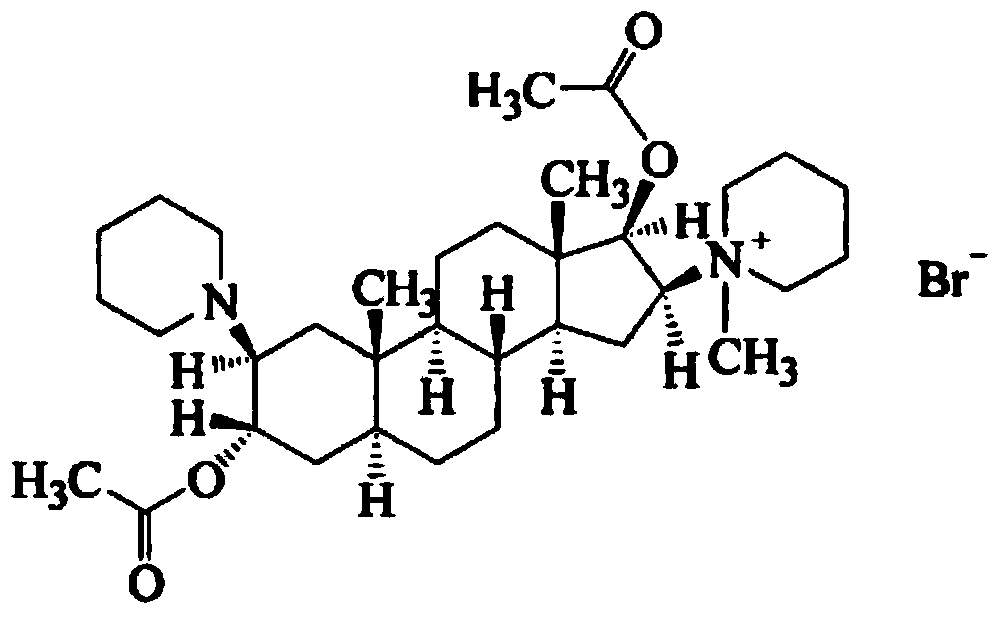
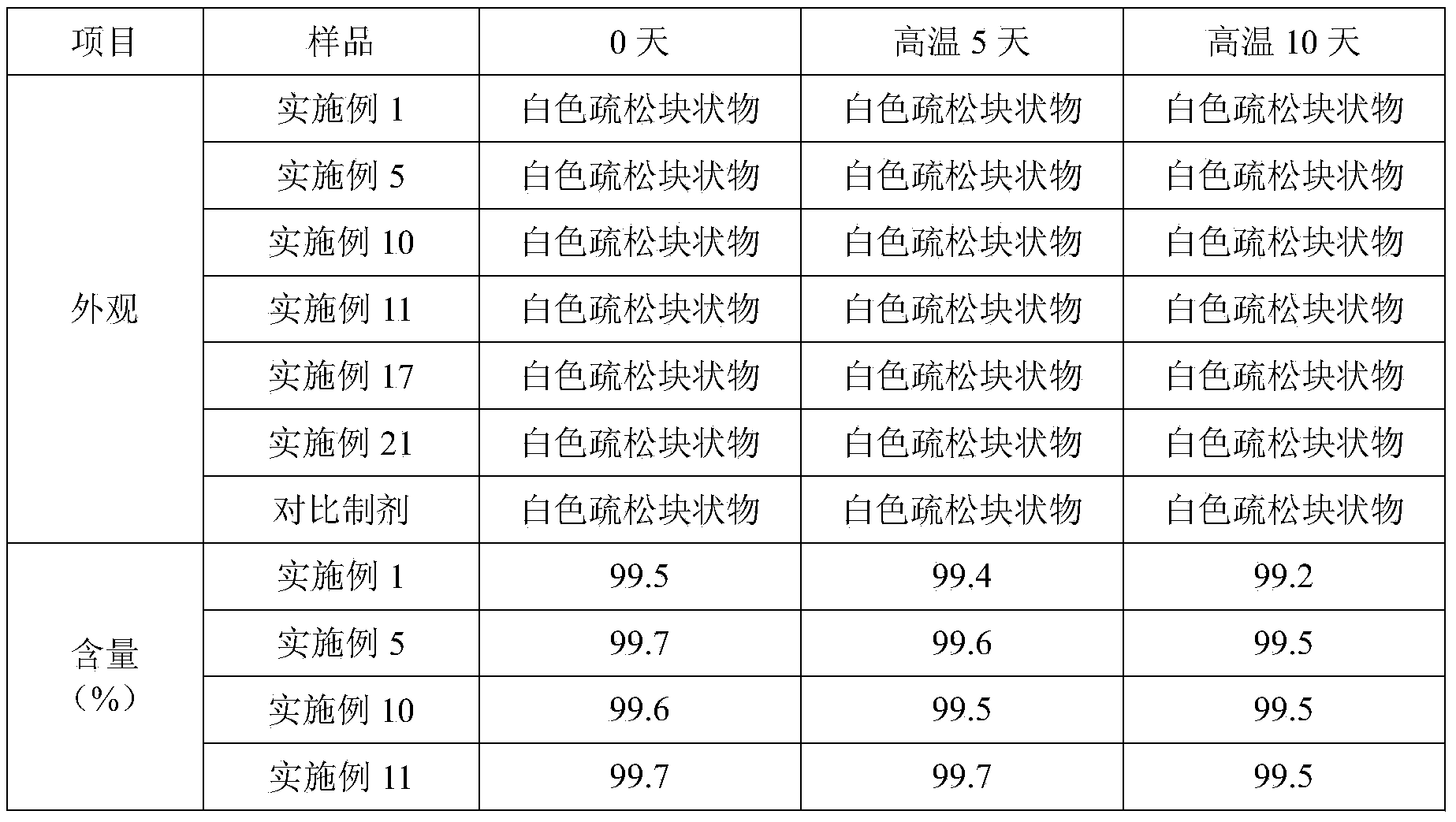
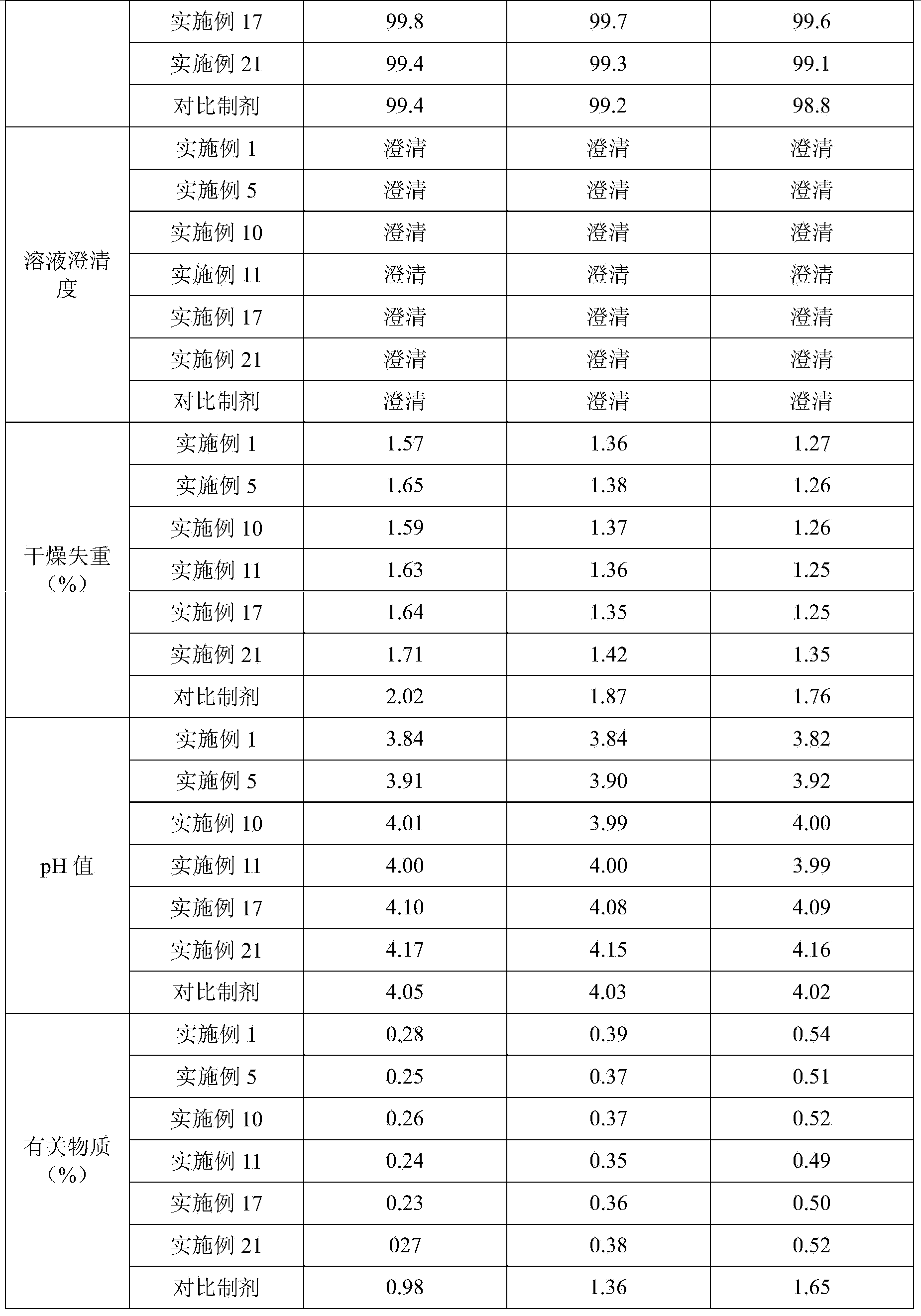
Vecuronium bromide pharmaceutical composition for injection and preparation method thereof
A technology of vecuronium bromide and a composition, applied in the field of medicine, can solve the problems of hidden safety hazards, high production cost, lactose intolerance and the like, and achieves the advantages of good safety, simple prescription and avoiding the phenomenon of lactose intolerance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Vecuronium Bromide Prescription for Injection (in 1000 bottles, unit: g)
[0046] Vecuronium Bromide
[0047] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, add 10g of citric acid, 8g of disodium hydrogen phosphate, and 2g of vecuronium bromide, heat to 50°C, stir until dissolved; add 0.1% (w / v) needle Use activated carbon, stir well, keep warm and adsorb for about 15 minutes, stir 2 to 3 times during this period, then filter for decarbonization, add water for injection to the full amount, and stir well; after fine filtration and clarification, conduct semi-finished product inspection, use phosphoric acid solution or Adjust the pH value with sodium solution, control the pH value at 3.8, measure the content of the solution, and fill it into a vial; freeze-dry after checking the difference in filling volume and clarity; capping and sealing; packaging. Example 2 Prescription of Vecuronium Bromide for Injection (...
Embodiment 8
[0060] Vecuronium Bromide
[0061] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, add 14g of citric acid, 11g of disodium hydrogen phosphate, and 3g of vecuronium bromide, heat to 50°C, stir until dissolved; add 0.1% (w / v) needle Use activated carbon, stir well, keep warm and adsorb for about 15 minutes, stir 2 to 3 times during this period, then filter for decarbonization, add water for injection to the full amount, and stir well; Adjust the pH value with sodium solution, control the pH value at 4.2, measure the content of the solution, and fill it into a vial; freeze-dry after checking the difference in filling volume and clarity; capping and sealing; packaging. Example 9 Prescription of Vecuronium Bromide for Injection (in 1000 bottles, unit: g)
[0062] Vecuronium Bromide
[0063] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, add 10g of citric acid, 8g of disodium hydrogen ph...
Embodiment 12
[0068] Vecuronium Bromide
[0069] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, add 14g of citric acid, 11g of disodium hydrogen phosphate, 4g of vecuronium bromide, heat to 50°C, stir until dissolved; add 0.1% (w / v) needle Use activated carbon, stir well, keep warm and adsorb for about 15 minutes, stir 2 to 3 times during this period, then filter for decarbonization, add water for injection to the full amount, and stir well; Adjust the pH value with sodium solution, control the pH value at 4.1, measure the content of the solution, and fill it into a vial; freeze-dry after checking the difference in filling volume and clarity; capping and sealing; packaging. Example 13 Prescription of Vecuronium Bromide for Injection (in 1000 bottles, unit: g)
[0070] Vecuronium Bromide
[0071] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, add 16g of citric acid, 12g of disodium hydrogen phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com