Fluorescence molecular probe and application thereof in hydrogen sulfide detection
A technology of fluorescent molecular probes and hydrogen sulfide, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of reducing the molecular performance of fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Synthesis of AIE active tetraphenylethylene azide derivatives:
[0047] Weigh diphenylmethane (5.5g, 32.8mmol) into a 500mL double-necked round-bottomed flask, place it in a liquid nitrogen acetone bath, evacuate nitrogen for three times, add 100mL freshly distilled tetrahydrofuran to dissolve, and slowly drop n-butyllithium ( 18.75mL, 30mmol), 4-bromobenzophenone (7.8g, 30mmol) was dissolved in an appropriate amount of freshly distilled tetrahydrofuran, added to the reaction system, and the system returned to room temperature overnight. The reaction was terminated with saturated ammonium chloride, and the crude product of intermediate 9 obtained after extraction was dissolved in an appropriate amount of toluene, 500 mg of p-toluenesulfonic acid was added, and refluxed overnight. The solvent was spin-dried, and the crude product obtained after extraction and washing was separated and purified by silica gel column chromatography (petroleum ether as eluent) to obtain ...
Embodiment 2
[0068] (1) The synthesis of tetraphenylethylene azide and aminotetraphenylethylene is the same as in Example 1
[0069] (2) Tetraphenylethylene azide qualitatively detects the presence of hydrogen sulfide as in Example 1
[0070] (3) The specificity and anti-interference identification of the detection of hydrogen sulfide by tetraphenylethylene azide in the aggregated state are the same as in Example 1.
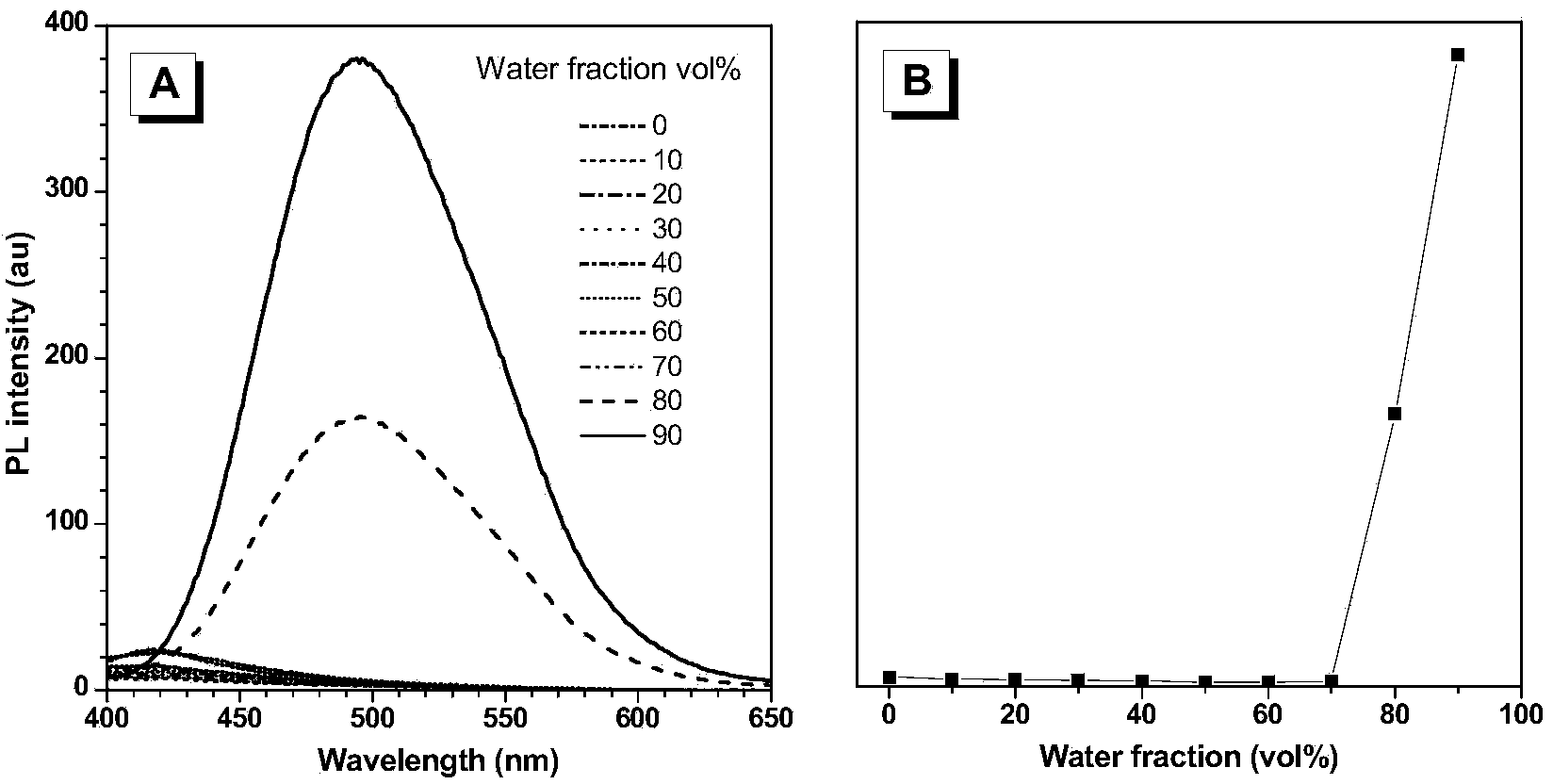
[0071] (4) Quantitative detection of hydrogen sulfide in aggregated state with different concentrations of tetraphenylethylene azide.
[0072] The quantitative detection process of hydrogen sulfide is as follows: prepare 2×10 -4 M tetraphenylethylene azide dimethyl sulfoxide solution, take 1mL and add it to a 10mL volumetric flask, then take 1mL dimethyl sulfoxide and add it to the volumetric flask, use a pipette to draw different volumes of 2×10 -2 Add the NaHS aqueous solution of M into the volumetric flask, adjust the pH to 7.4 with the HEPES buffer solution to set the v...
Embodiment 3
[0074] (1) The synthesis of tetraphenylethylene azide and aminotetraphenylethylene is the same as in Example 1
[0075] (2) Tetraphenylethylene azide qualitatively detects the presence of hydrogen sulfide as in Example 1
[0076] (3) The specificity and anti-interference identification of tetraphenylethylene azide in the aggregation state for the detection of hydrogen sulfide in this example are the same as those in Example 1.
[0077] (4) Quantitative detection of hydrogen sulfide in the aggregated state of tetraphenylethylene azide.
[0078] The quantitative detection process of hydrogen sulfide is as follows: prepare 5×10 -4 M tetraphenylethylene azide dimethyl sulfoxide solution, take 1mL and add it to a 10mL volumetric flask, then take 1mL dimethyl sulfoxide and add it to the volumetric flask, and use a pipette to draw different volumes of 5×10 -2 Add the NaHS aqueous solution of M into the volumetric flask, adjust the pH to 7.4 with the HEPES buffer solution to set the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com