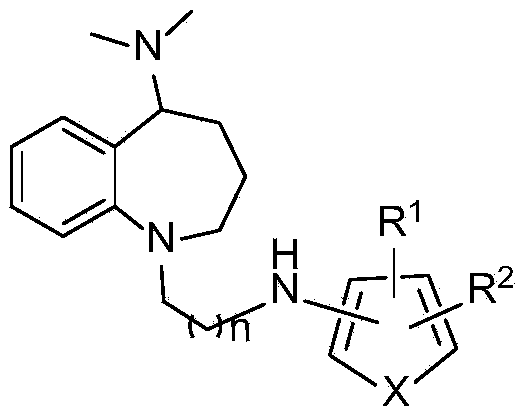
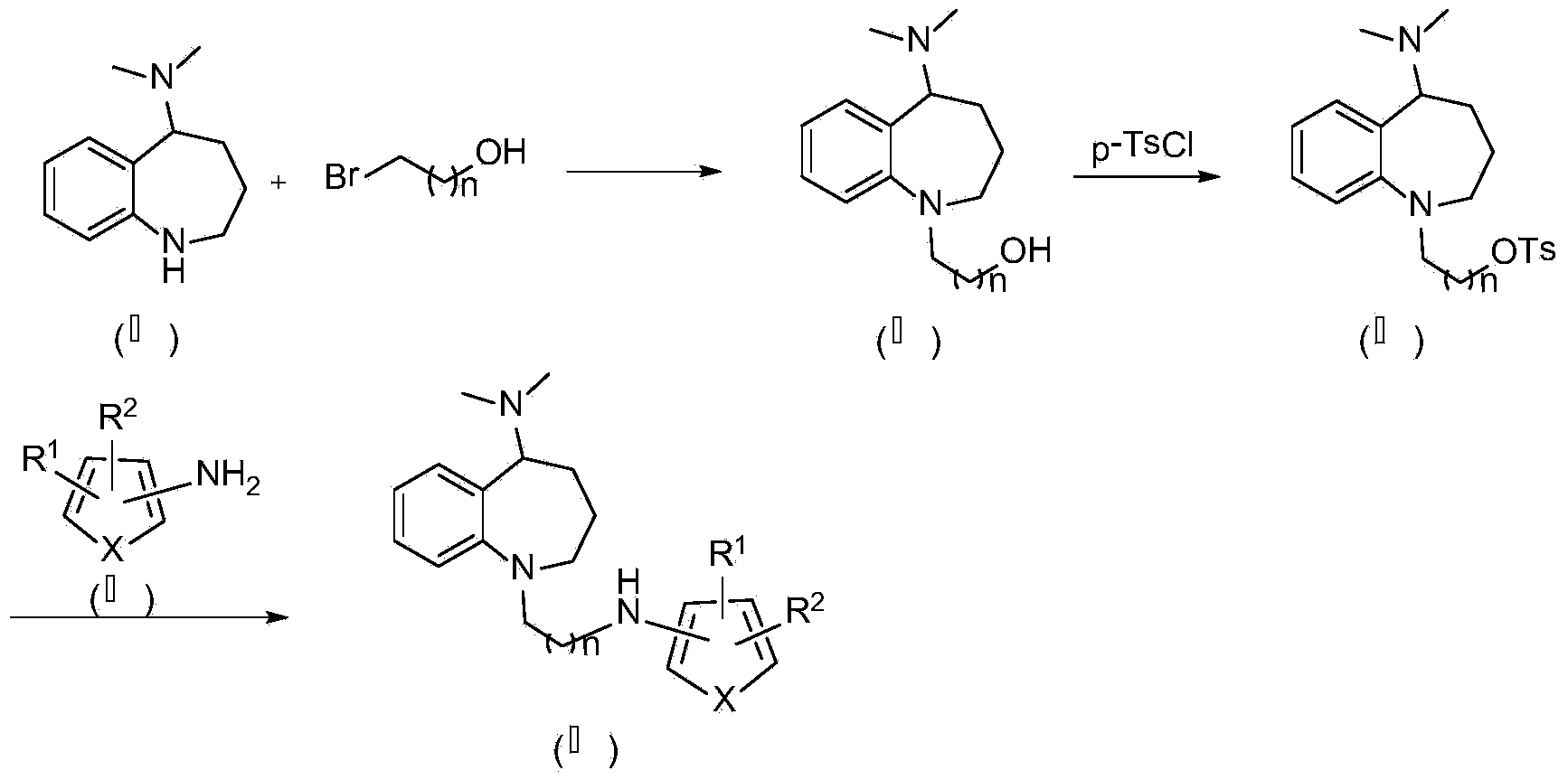
Benzoazepine derivative containing five-membered heterocycle as well as preparation method and application of derivative
A technology of phenyl and compound, applied in the field of compound with anti-tumor effect and its preparation, can solve the problems of damage, mutagenesis, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0047] Intermediate Ⅲ-2
[0048]
[0049] In a reaction flask equipped with stirring, condenser and thermometer, add 1.9g (0.010mol) N,N-dimethyl-2,3,4,5-tetrahydro-1H-benzo[b]azepine -5-amine (II), 2.0g (0.020mol) potassium bicarbonate and 30ml acetonitrile, add 2.78g (0.02mol) 3-bromopropanol dropwise under stirring, reflux reaction for 12h, TLC shows that the reaction is complete, filter the insoluble matter, The filtrate was poured into distilled water, extracted with ethyl acetate (15ml×3), the organic layers were combined, dried over anhydrous sodium sulfate, the solvent was evaporated, and the residue was separated by silica gel column chromatography to obtain a yellow solid with a yield of 82.5% and a purity of 97.5%. (HPLC normalization method), ESI-MS (m / z): 248.2.
Embodiment 3
[0051] Intermediate IV-1
[0052]
[0053] In a reaction flask equipped with stirring, condenser and thermometer, add 2.34g (0.01mol) of intermediate III-1, stir 50ml of pyridine to dissolve it, control the solution below -5°C, add 2.28g (0.012mol) in batches ) p-toluenesulfonyl chloride, reacted at 0°C for 10 hours, TLC showed that the reaction was complete, poured the reaction solution into cold water, solids precipitated, filtered, washed the filter cake with saturated brine (50ml×3), dried in vacuo, and obtained a white solid , yield 93.7%, purity 97.6% (HPLC normalization method), ESI-MS (m / z): 388.2.
[0054] Reference Example 4:
[0055] Intermediate Ⅳ-2
[0056]
[0057] Add 2.48g (0.01mol) of intermediate III-2, 50ml of N,N-dimethylformamide and 2.52g (0.025mol) of triethylamine into a reaction flask equipped with stirring, condenser and thermometer, and stir until It dissolves, and the solution is controlled below -5°C. Add 2.28g (0.012mol) p-toluenesulfon...
Embodiment 1
[0059] 3-(2-(5-(Dimethylamino)-2,3,4,5-tetrahydro-1H-benzo[b]azepine-1-yl)ethylamino)-1H-pyrrole- 2-Carboxylic acid ethyl ester (compound Ⅰ-1)
[0060]
[0061] In the reaction flask equipped with stirring, condenser and thermometer, add 3.88g (0.01mol) intermediate Ⅳ-1, 0.8g (0.02mol) sodium hydroxide, 30ml ethanol and 1.54g (0.01mol) 3-amino -1H-pyrrole-2-carboxylic acid ethyl ester, reflux reaction for 6h, TLC showed that the reaction was complete, filtered off the insoluble matter, evaporated the solvent, and the residue was separated by silica gel column chromatography to obtain compound Ⅰ-1: white solid, yield 78%, purity 99.4% (HPLC normalization method), HRMS(m / z)[M+H] + : 371.2442.
[0062] Example 2:
[0063] 2-(2-(5-(Dimethylamino)-2,3,4,5-tetrahydro-1H-benzo[b]azepine-1-yl)ethylamino)-1H-pyrrole- 3-carbonitrile (compound Ⅰ-2)
[0064]
[0065] In a reaction flask equipped with stirring, condenser and thermometer, add 3.88g (0.01mol) of intermediate IV-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com