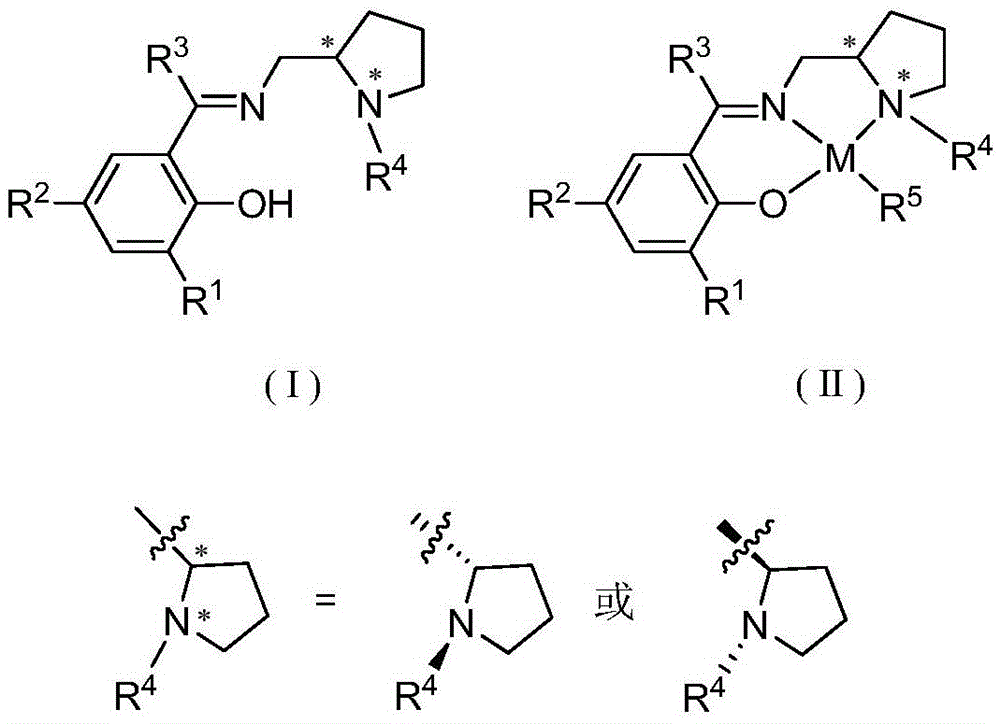
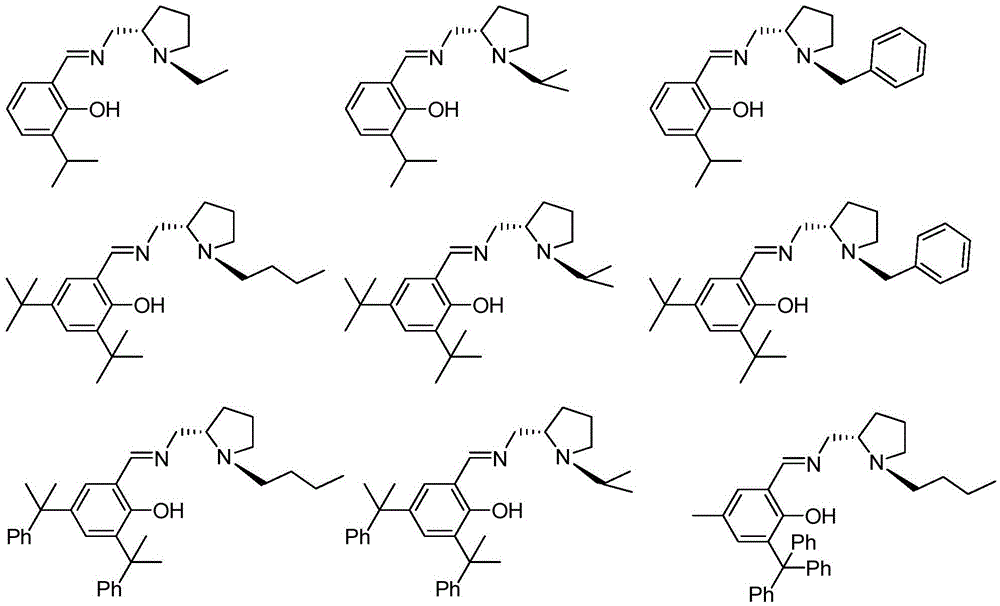
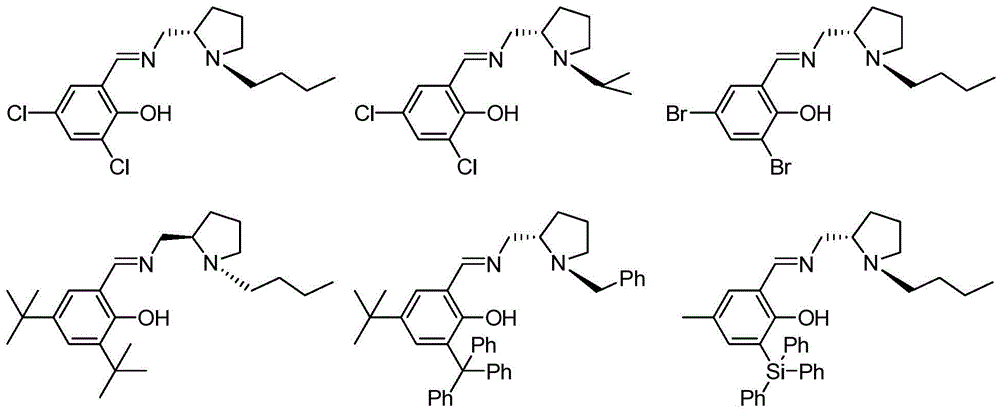
Chiral imine phenoxy zinc, magnesium compound and its preparation method and application
A technology of iminophenoxyzinc and magnesium compounds, applied in the field of magnesium compounds and chiral iminophenoxyzinc, can solve problems such as low selectivity and few catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Ligand L1
[0047] Add 1.65 g of 3-isopropyl salicylaldehyde, 30 mL of absolute ethanol, and 1.35 g of (S)-1-ethyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Dry over anhydrous magnesium sulfate, remove the solvent and excess low-boiling reactants to obtain a yellow viscous liquid. The pure product was obtained without further workup (2.51g, 91.6%).
[0048]
[0049] 1 HNMR (CDCl 3 ,400MHz):δ13.86(s,1H),8.36(s,1H),7.25(d,J=6.4Hz,1H),7.09(d,J=2.4Hz,1H),3.74(ddd,J= 11.8,4.1,0.9Hz,1H),3.36(dd,J=11.8,8.8Hz,1H),3.07(sept,1H),3.01–2.90(m,2H),2.56(dt,J=8.9,6.6Hz ,1H),2.01–1.77(m,2H),1.76–1.69(m,2H),1.45(s,9H),1.31(s,9H),1.15(d,J=6.5Hz,3H),1.05( d,J=6.4Hz,3H). 13 C{ 1 H}NMR (CDCl 3 ,100MHz): δ166.3,158.2,139.8,136.6,126.7,125.8,117.9,65.1,60.5,51.2,48.8,35.0,34.1,31.5,29.5,29.4,23.4,22.2,17.8.Anal.Calcd.ForC 17 h 26 N 2 O:C,74.41;H,9.55;N,10.21.Found:C,74.54;H,9.69;N,10.15%.
Embodiment 2
[0051] Synthesis of Ligand L2
[0052] Add 2.34g of 3,5-di-tert-butyl salicylaldehyde, 30mL of absolute ethanol, and 1.60g of (S)-1-butyl-2-aminomethyltetrahydropyrrole into a 100mL eggplant-shaped bottle, and heat to reflux for 24 hours . Dry over anhydrous magnesium sulfate, remove the solvent and excess low-boiling raw materials to obtain a yellow viscous liquid (3.58g, 96.1%).
[0053]
[0054] 1 HNMR (CDCl 3,400MHz):δ13.86(s,1H),8.36(s,1H),7.37(d,J=2.4Hz,1H),7.09(d,J=2.4Hz,1H),3.81(dd,J= 11.7,4.6Hz,1H),3.41(dd,J=11.7,7.8Hz,1H),3.21–3.12(m,1H),2.82(ddd,J=11.7,9.5,6.9Hz,1H),2.75–2.65 (m,1H),2.27(ddd,J=11.8,9.2,5.4Hz,0H),2.23–2.17(m,2H),1.96(ddd,J=16.1,12.1,8.2Hz,1H),1.85–1.78 (m,1H),1.78–1.70(m,2H),1.69–1.60(m,1H),1.57–1.46(m,2H),1.44(s,9H),1.31(s,9H),0.90(t ,J=7.3Hz,3H). 13 C{ 1 H}NMR (CDCl 3 ,100MHz): δ166.4,158.2,139.8,136.4,126.7,125.7,117.9,64.7,64.3,55.3,54.4,35.0,34.1,31.5,30.1,29.5,29.4,22.8,20.7,14.0.Anal.Calcd.ForC 24 h 39 N 2 O:C,77.58;H,10.58;N,7.5...
Embodiment 3
[0056] Synthesis of Ligand L3
[0057] Add 3.58 g of 3,5-dicumyl salicylaldehyde, 30 mL of absolute ethanol, and 1.60 g of (S)-1-butyl-2-aminomethyltetrahydropyrrole into a 100 mL eggplant-shaped bottle, and heat to reflux for 24 hours. Dry over anhydrous magnesium sulfate, remove the solvent and low-boiling raw materials to obtain a yellow viscous liquid (4.71g, 94.8%).
[0058]
[0059] 1 HNMR (CDCl 3 ,400MHz):δ13.45(s,1H),8.21(s,1H),7.31(d,J=2.4Hz,1H),7.28(s,1H),7.27(s,1H),7.26(s, 1H),7.23(s,1H),7.21(s,1H),7.19(d,J=4.6Hz,3H),7.16(d,J=1.9Hz,1H),7.10(d,J=6.8Hz, 1H),7.00(d,J=2.3Hz,1H),3.64(dd,J=11.7,4.7Hz,1H),3.27(dd,J=11.7,7.7Hz,1H),3.09(td,J=6.5 ,3.1Hz,1H),2.73–2.64(m,1H),2.63–2.53(m,1H),2.22–2.10(m,2H),1.82(dd,J=12.3,8.1Hz,1H),1.69( s,6H),1.66(s,3H),1.64(s,3H),1.55–1.44(m,2H),1.40(ddd,J=10.0,7.6,3.6Hz,2H),1.30–1.24(m, 2H),0.85(t,J=7.3Hz,3H). 13 C{ 1 H}NMR (CDCl 3 ,100MHz):165.9,157.9,150.8,150.7,139.3,136.0,128.8,128.0,127.7,126.7,125.6,125.0,118.0,64.4,64.4,55.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com