A recombinant human endostatin imaging agent and its preparation method
A technology of vascular endothelium and imaging agent, which is applied in the medical field and can solve the problems of low imaging resolution and limited clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
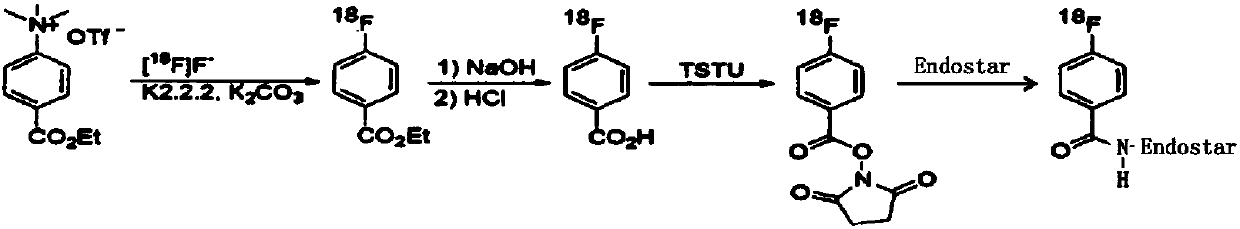
[0032] Example 1: 18 F-FB-Endostar labeling synthesis experiment
[0033] The synthesis steps are as follows:
[0034] 1. 800mCi synthesized by accelerator 18 F ion and 20mg K2.2.2, 5mg K 2 CO 3 React with 5 mg of ethyl 4-trimethylamine benzoate trifluoromethanesulfonate at 120°C for 20 min, then add 1 ml of 0.5 mmol / L NaOH solution for hydrolysis, then add 15 ml of 0.1 mmol / L HCL solution to generate 4- [ 18 F]fluorobenzoic acid ( 18 F-FBA);
[0035] 2. The foregoing 18 F-FBA was heated with 17mg TSTU (2-succinimidyl-1,1,3,3-tetramethyluronium tetrafluoroborate) for 15min, then added 3ml 5% acetic acid and 10mlHO 2 The acetic acid solution obtained by mixing O was acidified and purified by C-18 column to obtain 18 F-SFB ( 18 F-N-succinimide-4-fluorobenzoate).
[0036] 3. The above reaction obtained 18 F-SFB was eluted with acetonitrile, dried with nitrogen, and then 1mg Endostar dissolved in 0.5ml phosphate buffer (pH8.2) was added to the reaction tube, and reacte...
Embodiment 2
[0037] Example 2. Stability of markers in human serum
[0038] 500µCi will be obtained 18 F-FB-Endostar was mixed with 1ml of normal human serum and placed at 37°C. Samples were taken at 1h and 2h for R-TLC analysis (radioactive chromatography analysis) to observe the stability of the marker in human serum.
[0039] see results figure 2 Analysis, when mixed with normal human serum for 1 hour and 2 hours, the position of the radiation peak of the marker did not change, and no other radioactive peaks appeared. It shows that the marker has good stability in serum, and there is no obvious degradation in serum at 37°C for 2 hours.
Embodiment 3
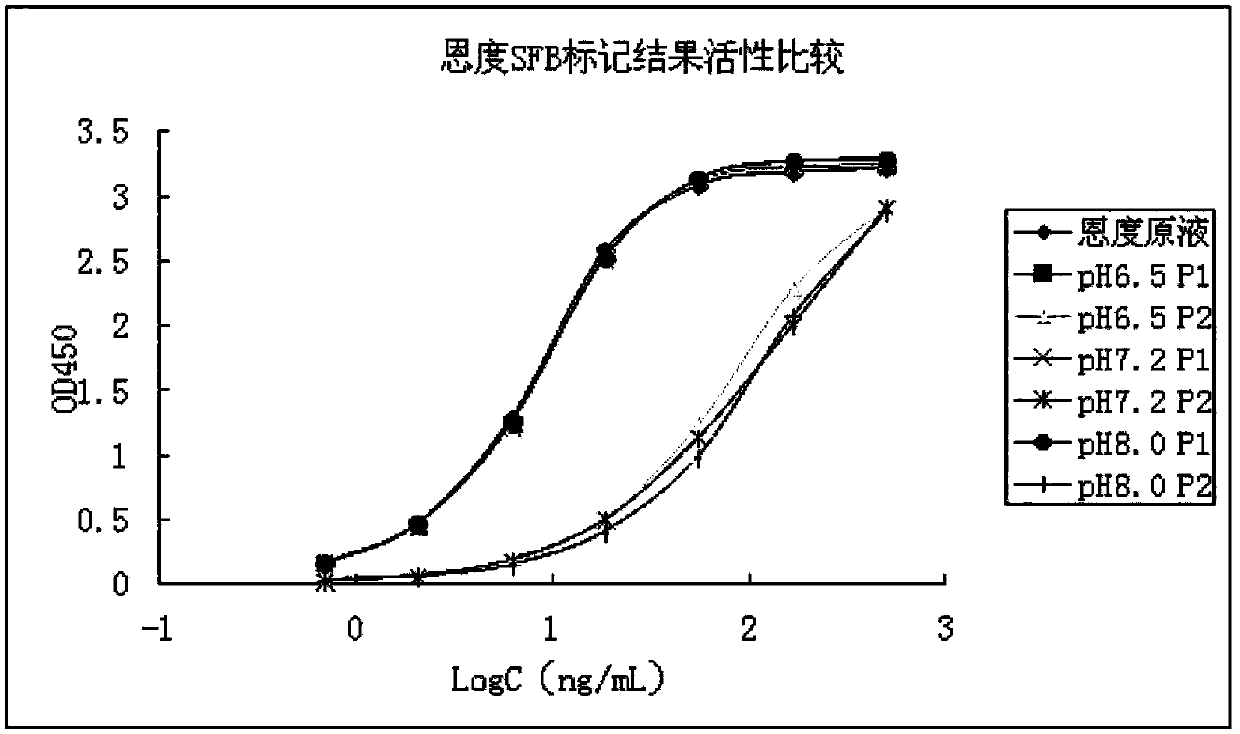
[0040] Embodiment 3. Different pH values carry out 19 F-SFB labeled Endostar experiment and detection of Endostar activity after labeling
[0041] In order to verify whether Endostar is still active after labeling, we carried out experiments under different pH conditions. 19 F-SFB labeled Endostar experiment and detection of Endostar activity after labeling. In order to avoid radioactive contamination when detecting activity, non-radioactive 19 F instead 18 F is marked, while 19 F and 18 F has exactly the same chemical properties. 19 F-SFB was synthesized by Changshu Huayi Chemical Co., Ltd.
[0042] Under the conditions of pH 6.5, 7.0 and 8.0 respectively, carry out 19 F-SFB with marking of Endostar, 19 The molar ratio of F-SFB to Endostar was 20:1, the reaction temperature was 37°C, and the reaction time was 30min. After the reaction was completed, the reaction product was eluted and separated by a desalting column, and two peaks were obtained during the eluted se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com