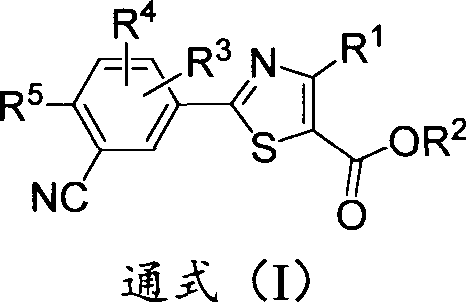
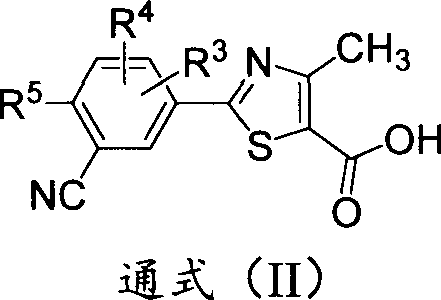
Deuterium-substituted 2-phenylthiazole compound, and pharmaceutical composition thereof
A compound, thiazole technology, is used as a xanthine oxidase inhibitor to treat gout and hyperuricemia, and can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
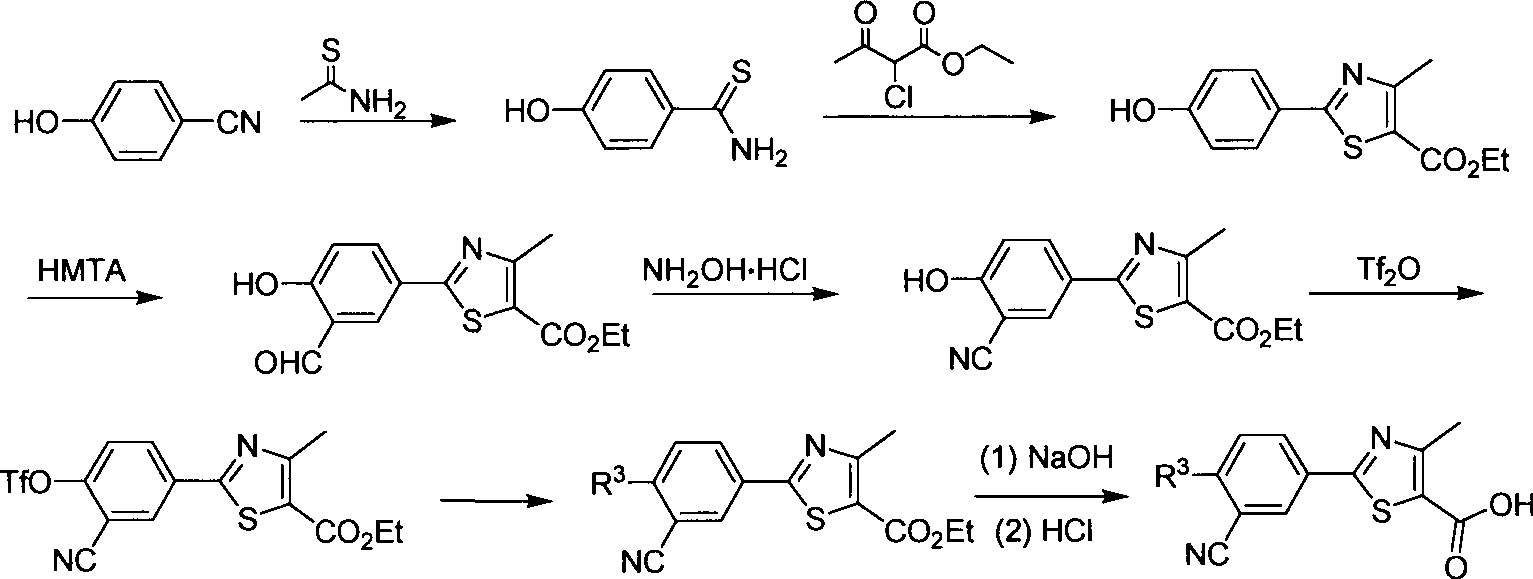
Embodiment 1
[0085] 2-[3-cyano-4-(2′,3′,4′,5′,6′-pentadeuteriophenyl)-phenyl]-4-methyl-thiazole-5-carboxylic acid (8) Synthesis
[0086]
[0087] Step A: Add thioacetamide (17.0g, 226.7mmol) and p-hydroxybenzonitrile (18.0g, 151.1mmol) into concentrated hydrochloric acid (470mL), heat up to 50°C, stir for 3 hours, TLC analysis shows that the reaction is complete . The reaction solution was cooled to 0°C, filtered, the filter cake was washed with a small amount of water, and dried in vacuo to obtain a crude product of p-hydroxythiobenzamide (2) (26.0g) as a yellow powder, which was directly used in the next step without purification . MS (EI, m / z): 154.1 [M+H] + .
[0088] Step B: The crude compound 2 (25.0g, 163.2mmol) and ethyl 2-chloroacetoacetate (26.5g, 161.0mmol) were added to absolute ethanol (75mL), and the resulting mixture was stirred at reflux for 3 hours and analyzed by TLC Indicates the end of the reaction. The reaction solution was cooled to 0°C, continued to stir for...
Embodiment 2
[0096] Synthesis of 2-(3-cyano-4-phenyl-5-deuterophenyl)-4-methyl-thiazole-5-carboxylic acid (14)
[0097]
[0098] Step A: NBS (4.2g, 23.6mmol) was added in batches to a mixture containing compound 5 (6.2g, 21.5mmol), methanol (150mL) and triethylamine (6mL) in an ice-water bath, and the addition was completed. Stirring was continued for 30 minutes, and TLC analysis showed that the reaction was complete. The solvent was evaporated under reduced pressure, water (60mL) was added, extracted with ethyl acetate (40mL×3), the combined organic phase was washed with 2M hydrochloric acid (30mL), the product was precipitated in ethyl acetate, filtered, and the filter cake was dried in vacuo Ethyl 2-(3-cyano-4-hydroxy-5-bromophenyl)-4-methyl-thiazole-5-carboxylate (9) (5.6 g) was obtained as a milky white solid. Yield: 82.3%.
[0099] 1 HNMR (DMSO-d 6 , 400MHz) δ8.34(d, J=2.4Hz, 1H), 8.22(d, J=2.0Hz, 1H), 4.29(q, J=7.2Hz, 2H), 2.65(s, 3H), 1.30( d, J=6.8Hz, 3H). MS (EI, m / z): 3...
Embodiment 3
[0108] Synthesis of 2-[3-cyano-4-(4-methylphenyl)-5-deuterophenyl]-4-methyl-thiazole-5-carboxylic acid (16)
[0109]
[0110] For the experimental operation, see Step E and Step F in Example 2.
[0111] 1 HNMR (DMSO-d 6 , 400MHz) δ8.46(s, 1H), 8.31(s, 1H), 7.55(d, J=8.0Hz, 2H), 7.38(d, J=8.0Hz, 2H), 2.70(s, 3H), 2.41(s, 3H). MS (EI, m / z): 335.2 [M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com