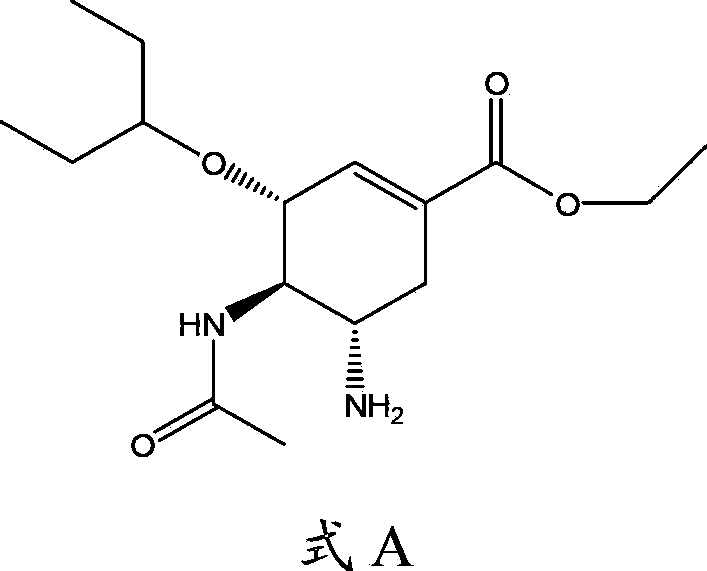
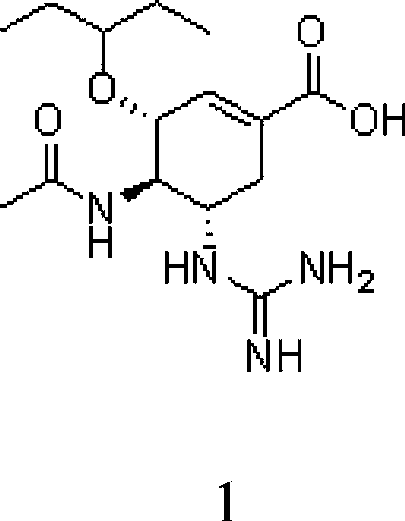
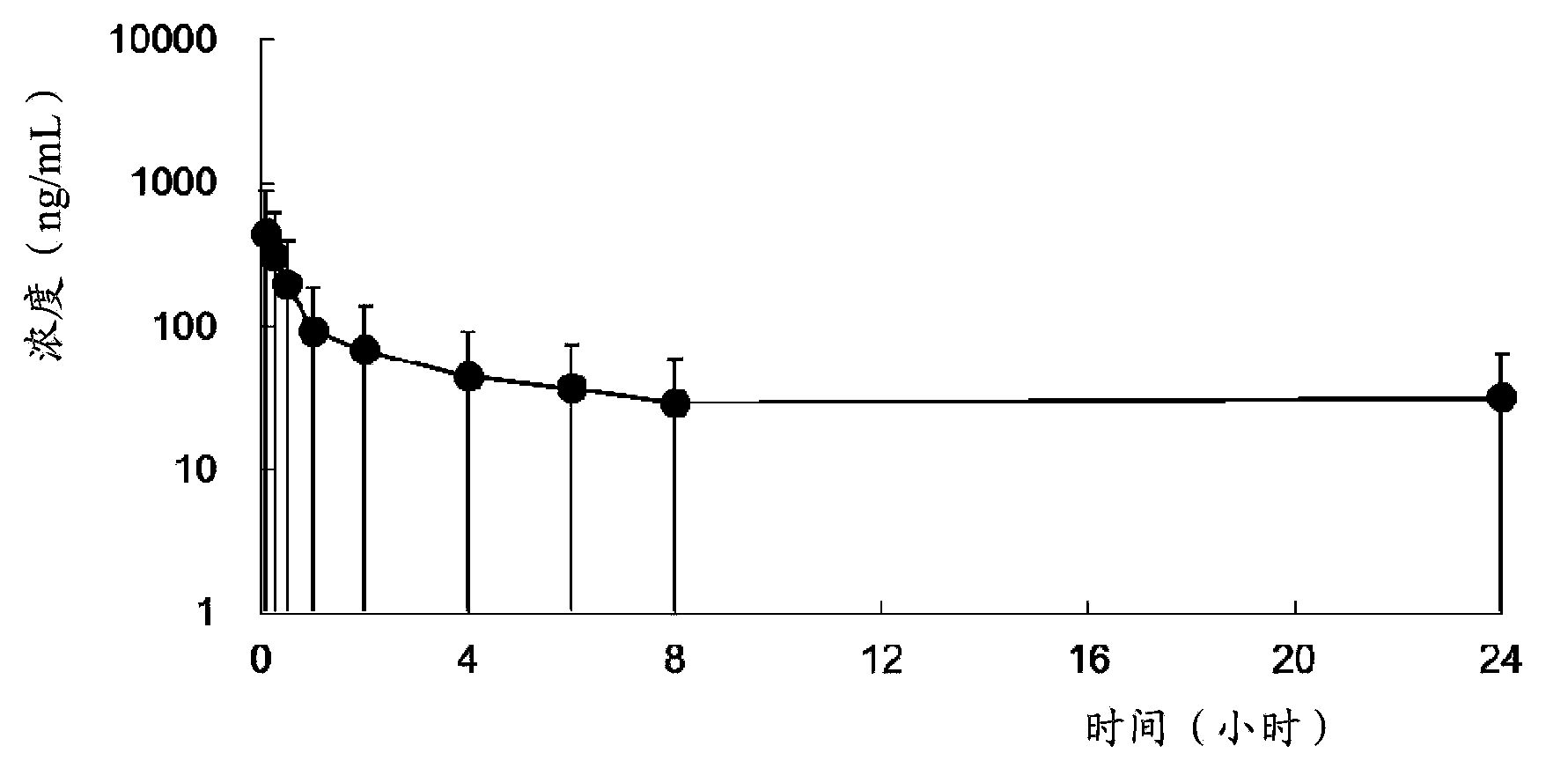Use of neuraminidase inhibitor and neuraminidase inhibitor prodrug
A use and prodrug technology, applied in the preparation of organic compounds, antiviral agents, pharmaceutical formulations, etc., can solve the problems of drug resistance and Tamiflu’s inability to exert antiviral effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of compound 1
[0045] Synthetic scheme
[0046]
[0047] 1. Synthesis of Compound 1-10
[0048]
[0049] To a 250-mL 3-neck round-bottom flask was added a solution of thiourea (2 g, 26.27 mmol, 1.00 equiv) in THF (50 mL), then NaH (60%) was added in several portions with stirring at -10-0 °C (2.3g). The resulting solution was stirred at -10-0 °C for 1 h. A solution of di-tert-butyl dicarbonate (12.6 g, 57.73 mmol, 2.20 equiv) in tetrahydrofuran (50 mL) was added dropwise thereto with stirring at 0°C. The resulting solution was stirred overnight at room temperature, quenched by the addition of 100 mL of water, then extracted with 2x100 mL of ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, then concentrated under vacuum. Purification of the residue on a silica gel column eluting with ethyl acetate:petroleum ether (1:5-1:2) afforded 1.5 g (21%) of tert-butyl N-([[(tert-butoxy)carbonyl] Amino]...
Embodiment 2
[0063] Embodiment 2: the preparation of compound I2
[0064] Synthetic scheme
[0065]
[0066] 1. Synthesis of compound I2-10
[0067]
[0068] To oseltamivir (4.1g, 10mmol, 1.0eq) and Et 3 To a mixture of N (6.1 g, 60 mmol, 6.0 eq) in EtOH (10 mL) was added BrCN (1.1 g, 10 mmol). The mixture was then stirred overnight at room temperature. TLC and LCMS showed the reaction was complete. To the resulting mixture was added brine (50 mL). The mixture was concentrated under vacuum to remove EtOH, then the residue was extracted with DCM (50 mL x 3). The extract was washed with brine (50 mL), washed with Na 2 Drying over SO4 and concentration gave the crude product (3.9 g) as a yellow oil. The residue was used in the next step without purification.
[0069] 2. Synthesis of compound I2
[0070]
[0071] To a solution of compound I2-10 (9 g) and Et3N (8.1 g, 80.02 mmol, 3.0 eq) in EtOH (300 mL) was added NH2OH.HCl (11 g, 80.02 mmol, 3.0 eq). The mixture was stirre...
Embodiment 3
[0075] Embodiment 3: the preparation of compound I3
[0076] Synthetic scheme
[0077]
[0078] 1. Synthesis of compound I3-10
[0079]
[0080]To a 250-mL round bottom flask was added ethyl (3R,4R,5S)-5-amino-4-acetylamino-3-(pent-3-yloxy)cyclohex-1-ene-1-carboxylate ( 5 g, 16.00 mmol, 1.00 equiv), KOCN (4 g), acetic acid (50 mL), and water (50 mL). The resulting solution was stirred overnight at 50 °C, concentrated in vacuo, then washed with 100 ml H 2 O dilution. The solid was collected by filtration to yield 1.7 g (30%) of (3R,4R,5S)-5-(carbamoyl)-4-acetylamino-3-(pent-3-yloxy)cyclohex-1-ene -1-Ethyl carboxylate as a white solid.
[0081] 2. Synthesis of compound I3-11
[0082]
[0083] Into a 50-mL round bottom flask was added (3R,4R,5S)-5-(carbamoyl)-4-acetylamino-3-(pent-3-yloxy)cyclohex-1-ene-1- A solution of ethyl formate (500 mg, 1.41 mmol, 1.00 equiv) in pyridine (10 mL) and TsCl (1 g, 5.25 mmol, 3.73 equiv). The resulting solution was stirred ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com