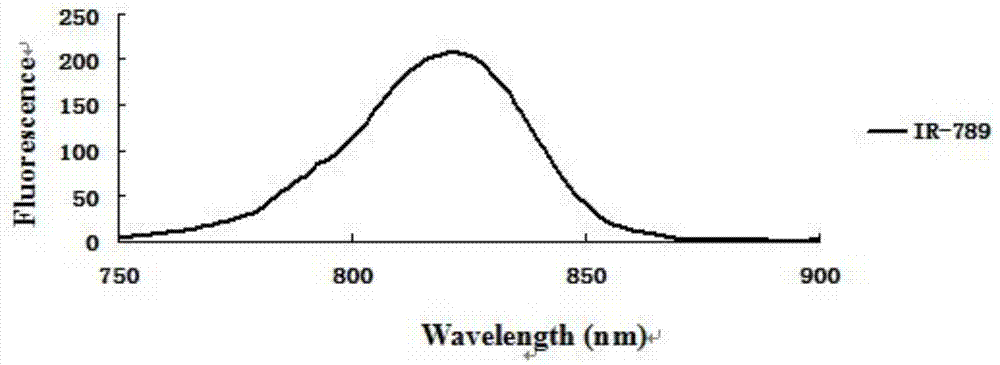
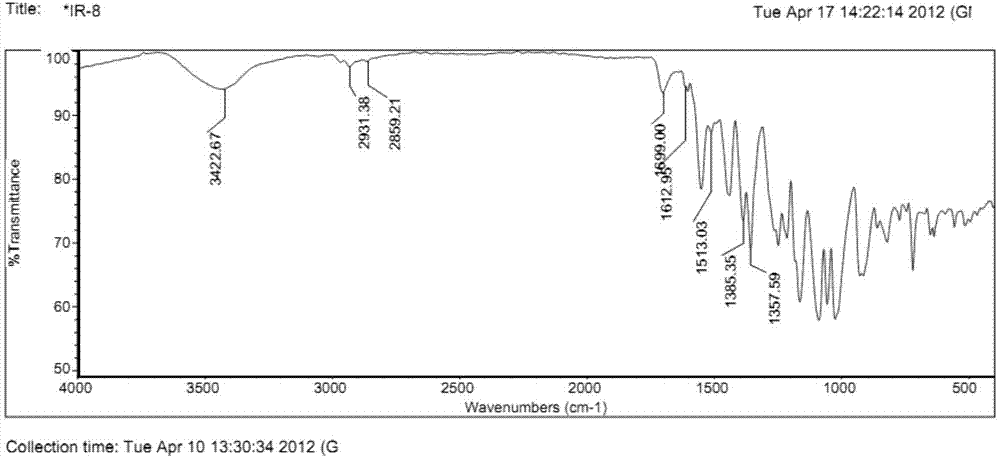
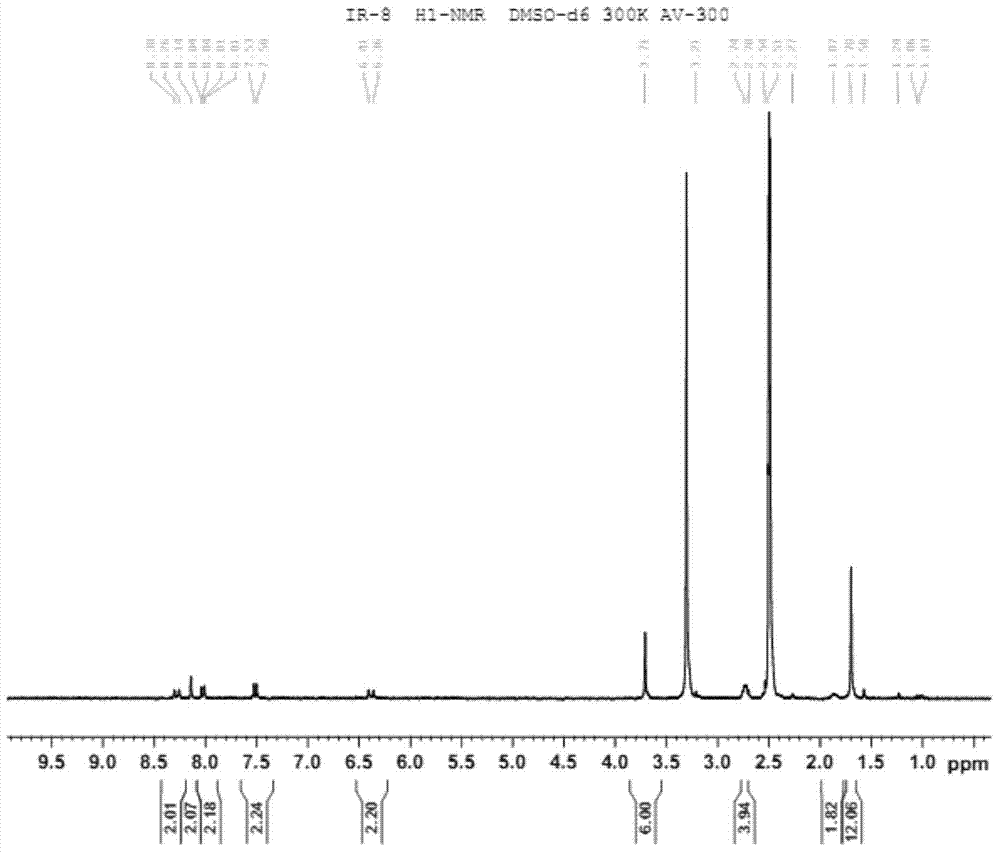
Near infrared fluorescence molecular probe, and preparation method and application thereof
A fluorescent molecular probe and near-infrared technology, applied in the field of biochemical detection, can solve the problems of difficult separation and purification of products, many side reactions of synthesis, etc., and achieve the effects of high sensitivity, good optical stability, and avoiding interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 0.25mmol of compound 5 into a 100mL reaction flask, add 50mL of anhydrous tetrahydrofuran (THF) and stir to dissolve, under the protection of argon, add 0.30mmol of compound 4 dropwise at 0°C, stir at 0°C for 2h, and the reaction ends. The product precipitated from the system, was filtered and dried to obtain 0.11 g of a white powdery solid. Yield: 61.11%. Mp=247-248°C.
[0042] Take 0.54mmol of compound 3 into a 50mL reaction flask, add 20mL of acetic anhydride and stir to dissolve, add 0.27mmol of compound 2 and 0.54mmol of anhydrous sodium acetate under the protection of argon, react at 30°C for 10h, and the reaction is completed. The reaction solution was poured into 100mL of methyl tert-butyl ether, and a green solid was precipitated, separated and purified by 200-300 mesh silica gel column chromatography, and the eluent was a mixture of dichloromethane and methanol at a volume ratio of 4:1 to obtain 55mg The green metallic color crystal is a near-infrared fl...
Embodiment 2
[0049] Add 0.25mmol of compound 5 into a 100mL reaction flask, add 50mL of anhydrous tetrahydrofuran (THF) and stir to dissolve, under the protection of argon, add 0.60mmol of compound 4 dropwise at 0°C, stir at 0°C for 3h, and the reaction ends. The product precipitated from the system, was filtered and dried to obtain 0.10 g of white powdery solid. . Mp=247-248°C.
[0050] Take 0.54mmol of compound 3 into a 50mL reaction flask, add 20mL of acetic anhydride and stir to dissolve, add 0.27mmol of compound 2 and 0.54mmol of anhydrous sodium acetate under the protection of argon, react at 20°C for 10h, and the reaction is completed. The reaction solution was poured into 100mL ether, and a green solid was precipitated, which was separated and purified by 200-300 mesh silica gel column chromatography. The eluent was a mixture of dichloromethane and methanol at a volume ratio of 8:1, and 53 mg of green metallic color crystals were obtained. Near-infrared fluorescent molecular prob...
Embodiment 3
[0052] Add 0.25mmol of compound 5 into a 100mL reaction flask, add 50mL of anhydrous tetrahydrofuran (THF) and stir to dissolve, under the protection of argon, add 0.90mmol of compound 4 dropwise at 0°C, stir at 0°C for 2h, and the reaction ends. The product precipitated from the system, was filtered and dried to obtain 0.105 g of white powdery solid.
[0053] Take 0.54mmol of compound 3 into a 50mL reaction flask, add 20mL of acetic anhydride and stir to dissolve, add 0.27mmol of compound 2 and 0.54mmol of anhydrous sodium acetate under the protection of argon, react at 30°C for 12h, and the reaction is completed. The reaction solution was poured into 100mL ether, and a green solid was precipitated, which was separated and purified by 200-300 mesh silica gel column chromatography. The eluent was a mixture of ethyl acetate and methanol at a volume ratio of 3:1, and 53.5 mg of green metallic color crystals were obtained. It is a near-infrared fluorescent molecular probe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com