Method for preparing N-(9-fluorenylmethoxy carbony)-O-tertiary butyl-L-tyrosine
A technology of fluorenylmethoxycarbonyl and tyrosine, which is applied in the field of preparation of N--O-tert-butyl-L-tyrosine, can solve the problem of harsh synthesis conditions of tyrosine, high energy consumption per unit product, and product recovery Low yield and other problems, to achieve the effect of convenient acquisition, low equipment requirements and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
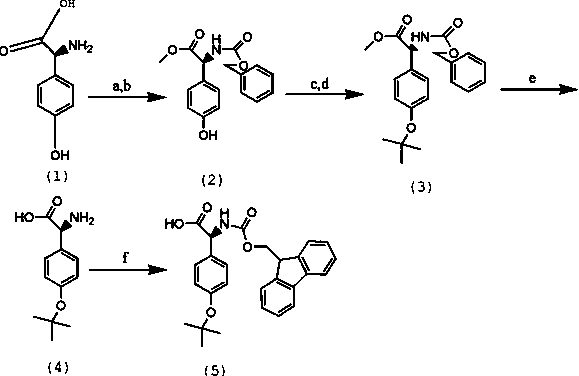
[0051] Embodiment 1, with reference to synthetic route:
[0052] 1. Add 20g of L-Tyr (tyrosine) and 600g of methanol into the reaction flask, add SOCl dropwise under stirring 2
[0053] (thionyl chloride) 100g, reflux reaction. When TLC (thin layer chromatography) detects that there is no L-Tyr (tyrosine) in the reaction system, the reaction is complete and Tyr-OMe·HCl (tyrosine hydrochloride) is obtained. The reaction solution after the above reaction was concentrated to dryness with hot water in vacuum at a temperature of 60°C.
[0054] 2. Add AcOEt (ethyl acetate) to the solid Tyr-OMe HCl (tyrosine hydrochloride) obtained above
[0055] ester) 300g, add Na under stirring 2 CO 3 (sodium carbonate) 100g, add water 50g, slowly drop Z-Cl (benzyl chloroformate) 230g. Control the pH of the system to 8, and when there is no Tyr-OMe HCl (tyrosine hydrochloride) in the reaction system detected by TLC (thin layer chromatography), then add citric acid, acidify to pH = 3, and sta...
Embodiment 2
[0064] Embodiment 2, with reference to synthetic route:
[0065] 1. Add 20g of L-Tyr (tyrosine) and 600g of methanol into the reaction flask, add SOCl dropwise under stirring 2
[0066] (thionyl chloride) 100g, reflux reaction. When TLC (thin layer chromatography) detects that there is no L-Tyr (tyrosine) in the reaction system, the reaction is complete and Tyr-OMe·HCl (tyrosine hydrochloride) is obtained. The reaction solution after the above reaction was concentrated to dryness with hot water in vacuum at a temperature of 60°C.
[0067] 2. Add AcOEt (ethyl acetate) to the solid Tyr-OMe HCl (tyrosine hydrochloride) obtained above
[0068] ester) 300g, add Na under stirring 2 CO 3 (sodium carbonate) 100g, add water 50g, slowly drop Z-Cl (benzyl chloroformate) 230g. Control system pH = 9, when TLC (thin layer chromatography) detects that there is no Tyr-OMe HCl (tyrosine hydrochloride) in the reaction system, then add citric acid, acidify to pH = 3, and statically separat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com