Medical application of 5-(1, 2, 4-triazole-1-yl)-2-phenylacetyl aminothiazole
A technology of phenylacetamidothiazole and alkyl, applied in the field of compound preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-phenylacetylaminothiazole:
[0016] 2.0 mmol 4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-amine was dissolved in 20.0 mL dichloromethane, 2.2 mmol phenylacetic acid, 0.03 g 4-dimethyl Aminopyridine (DMAP), 2.2 mmol N, N'-dicyclohexylcarbodiimide (DCC) was added after 0.5 h, stirred at room temperature, reacted for 6.0 h, the reaction solution was neutralized with aqueous sodium bicarbonate solution, stood still, and separated , the organic layer was dried with anhydrous sodium sulfate, filtered, rotary evaporated, and column chromatographed to obtain 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-phenylacetylaminothiazole. The rate is 58.1%, m.p. 177~180℃. 1 H NMR (400 MHz, CDCl 3 ) δ: 1.09 (s, 9H, 3×CH 3 ), 3.85 (s, 2H, CH 2 ), 7.33 (d, J = 7.2 Hz, 2H, C 6 h 5 2,6-H), 7.38-7.45 (m, 3H, C 6 h 5 3,4,5-H), 8.11 (s, 1H, C 2 N 3 h 2 3-H), 8.25 (s, 1H, C 2 N 3 h 2 5-H), 9.03 (s, 1H, NH).
Embodiment 2
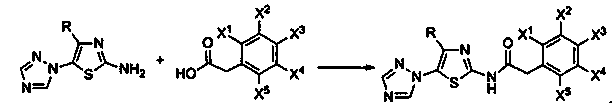
[0018] Preparation of 5-(1,2,4-triazol-1-yl)-2-phenylacetamidothiazole (I):
[0019]
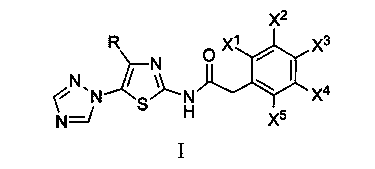
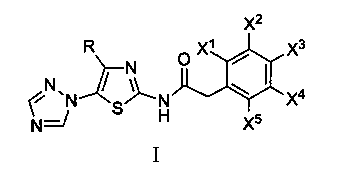
[0020] In the formula, R is selected from: C 1 ~C 2 Alkyl, C 3 ~C 4 Straight chain or branched chain alkyl; X 1 、X 2 、X 3 、X 4 、X 5 Respectively selected from: hydrogen, deuterium, C 1 ~C 2 Alkyl, C 3 ~C 4 Straight chain or branched chain alkyl
[0021] According to the method of Example 1, 5-(1,2,4-triazol-1-yl)-2-phenylacetamidothiazole represented by chemical structure I was prepared.
Embodiment 3
[0023] Anti-influenza virus neuraminidase activity of 5-(1,2,4-triazol-1-yl)-2-phenylacetylaminothiazole
[0024] 1. Experimental principle
[0025] The compound MUNANA is a specific substrate of neuraminidase. The metabolites produced under the action of neuraminidase can produce 450 nm fluorescence under the excitation of 360 nm irradiation, and the change of fluorescence intensity can sensitively reflect the activity of neuraminidase . Enzymes are all from A / PR / 8 / 34 (H1N1) virus strain
[0026] 2. Experimental method
[0027] In the enzyme reaction system, a certain concentration of samples and influenza virus neuraminidase NA are suspended in the reaction buffer (pH 6.5), the fluorescent substrate MUNANA is added to start the reaction system, and after incubation at 37°C for 40 minutes, the reaction termination solution is added to terminate the reaction. reaction. Fluorescence intensity values were measured under the parameter conditions of excitation wavelength 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com