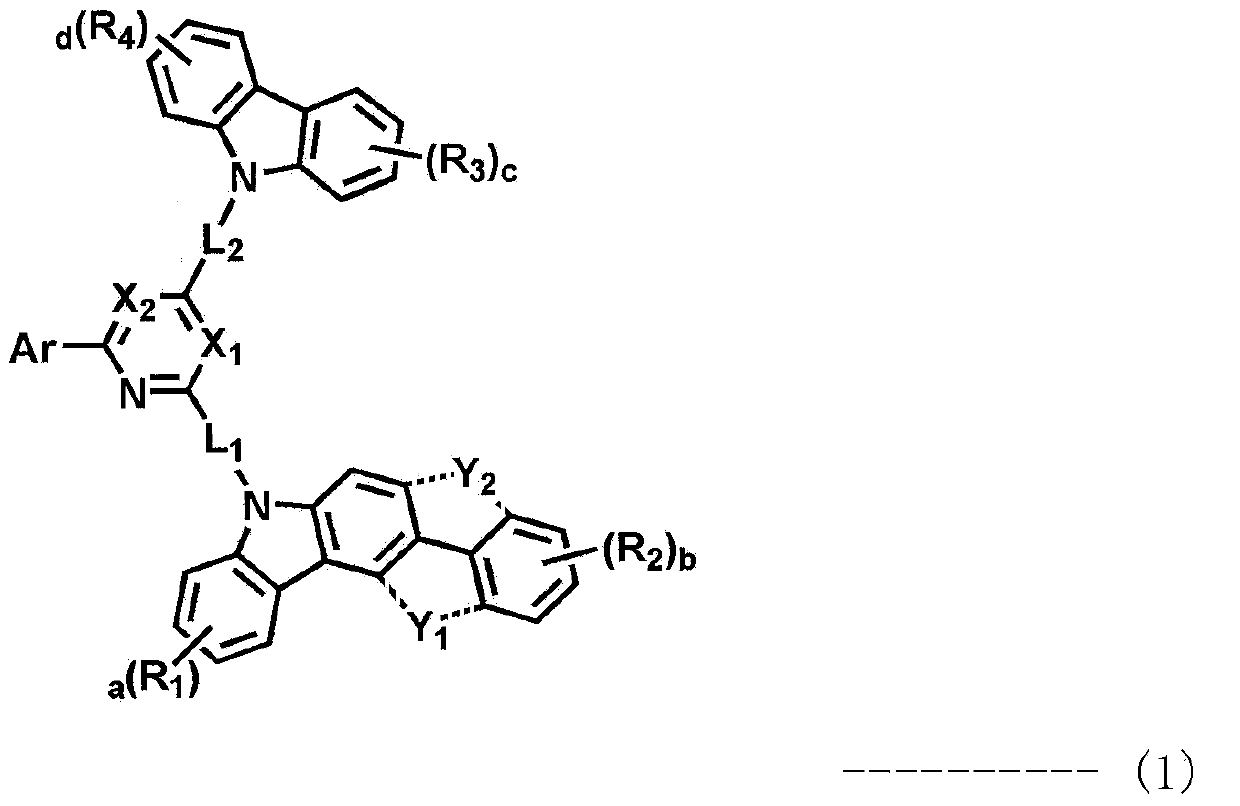
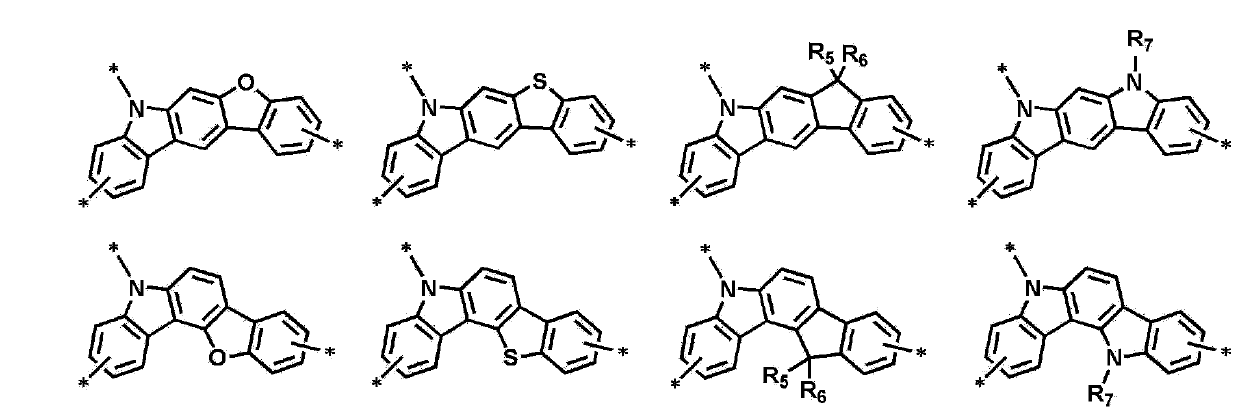
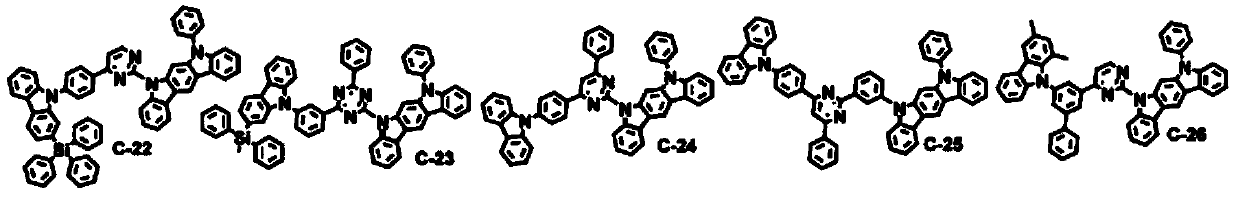
Novel organic electroluminescence compounds and organic electroluminescence device using the same
A compound and unsubstituted technology, applied in the field of organic electroluminescent devices, can solve the problems of no disclosure of carbazole compounds, low glass transition temperature, poor thermal stability, etc., and achieve improved current characteristics, low driving voltage, good layering sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the preparation of compound C-40
[0073]
[0074] Preparation of Compound 1-1
[0075] 2-Bromo-9,9-dimethyl-9H-fluorene (50 g, 183.0 mmol), 2-chloroaniline (38 mL, 366.1 mmol), Pd(OAc) 2 (1.2 g, 5.5 mmol), P(t-Bu) 3 (8.8 mL, 18.3 mmol), NaOt-Bu (35 g, 366.1 mmol) and toluene (180 mL) were mixed, and the reaction mixture was stirred at reflux for 2.5 hours. After terminating the reaction, the reaction mixture was filtered, and the filter cake was washed with dichloromethane. The resulting organic layer was washed with pure water and washed with MgSO 4 Dry and concentrate under reduced pressure. Then, the crude oil was filtered through silica gel, and the remaining solution was concentrated under reduced pressure to obtain Compound 1-1 (47 g, 80%).
[0076] Preparation of compound 1-2
[0077] Compound 1-1 (46 g, 143.8 mmol), Pd(OAc) 2 (968 mg, 4.3 mmol), di-tert-butyl(methyl)phosphorus tetrafluoroborate (2 g, 4.31 mmol) and DMAc (200 mL) wer...
Embodiment 2
[0083] Embodiment 2: the preparation of compound C-94
[0084]
[0085] Preparation of compound 2-1
[0086] 1-Bromo-2-nitrobenzene (85 g, 0.42 mol), dibenzo[b,d]thiophen-2-ylboronic acid (80 g, 0.35 mol), Pd(PPh 3 ) 4 (20g, 0.018mol), K 2 CO 3 (116 g, 1.0 mol), toluene (1700 mL), EtOH (440 mL), and distilled water (440 mL) were mixed, and the reaction mixture was stirred at 120° C. for 12 hours. After the reaction was terminated, the reaction mixture was extracted with EA. with MgSO 4 The resulting organic layer was dried, filtered, evaporated under reduced pressure to remove the solvent, and filtered through a column to obtain compound 2-1 (93 g, 87%).
[0087] Preparation of Compound 2-2
[0088] Compound 2-1 (88 g, 0.29 mol), P(OEt) 3 (960 mL, 0.4M) and triethyl phosphite (960 mL) were mixed, and the reaction mixture was stirred at 90° C. for 12 hours. After terminating the reaction, the reaction mixture was distilled to remove triethyl phosphite, and fil...
Embodiment 3
[0092] Embodiment 3: the preparation of compound C-105
[0093]
[0094] Preparation of compound 3-1
[0095] Carbazole (26 g, 155.49 mmol), 4-bromoiodobenzene (87 g, 310.9 mmol), CuI (14.8 g, 77.74 mmol), ethylenediamine (9.3 g, 155.49 mmol), K 3 PO 4 (99 g, 499.47 mmol) and toluene (1000 ml) were mixed, and the reaction mixture was stirred at reflux. After 15 h, the reaction mixture was cooled to room temperature and filtered under reduced pressure to remove CuI and K 3 PO 4 . The remaining solution was extracted with MC, washed with distilled water, and then filtered through a column to obtain Compound 3-1 (35 g, 70%).
[0096] Preparation of Compound 3-2
[0097] After compound 3-1 (35 g, 108.6 mmol) was dissolved in THF (600 mL), n-BuLi (52 mL, 130.35 mmol, 2.5 M in hexane) was added to the reaction mixture at -78 °C , and the reaction mixture was stirred for 1 h. The reaction mixture was stirred at room temperature for 12 hours and B(Oi-Pr) was slowly ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com