Ginsenoside Rg1-phenyl isocyanate chiral stationary phase filler and preparation method thereof
A technology of phenyl isocyanate and chiral stationary phase, which is applied in the field of analytical chemistry, achieves the effect of simple preparation method and meeting the needs of daily drug analysis and production quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0029] In this example, a natural product-ginsenoside Rg1 is used as a raw material, chemically bonded to a silica gel carrier silanized with 3-(aminopropyl)-triethoxysilane through a spacer arm, and then derivatized with benzene isocyanate to prepare to make.
[0030] The specific separation parameters of the amino acid in the reversed-phase mobile phase are as follows:
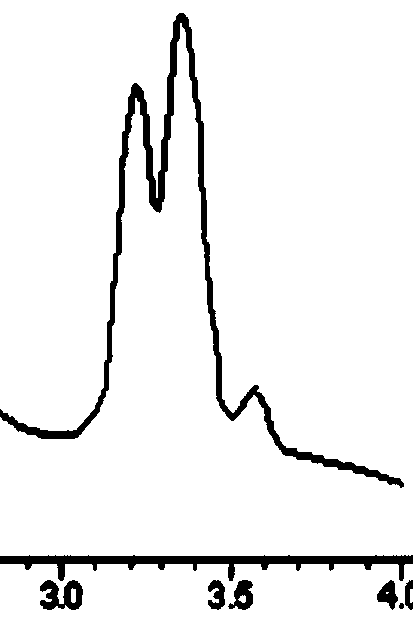
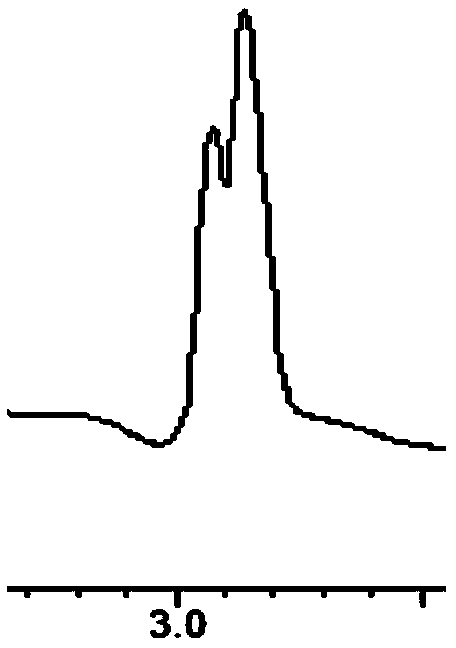
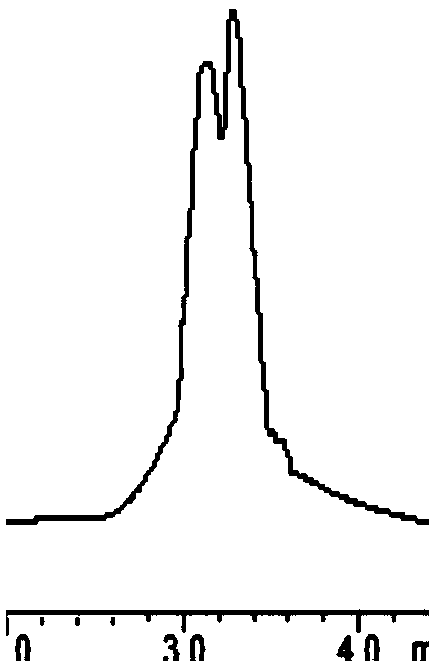
[0031] The mobile phase was acetonitrile / 0.05% glacial acetic acid-triethylamine buffer salt=90 / 10. The flow rate is 0.4mL / min, and the separation results are shown in the following table and Figures 1 to 9 ; Detection wavelength is 210nm.
[0032] The table shows the separation results of ginsenoside Rg1-phenyl isocyanate chiral immobilization relative amino acid enantiomers in reversed-phase mobile phase:
[0033]
[0034]
[0035] Mobile phase: acetonitrile / 0.05% glacial acetic acid-triethylamine buffer salt=90 / 10. The flow rate was 0.4 mL / min, and the detection wavelength was 210 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com