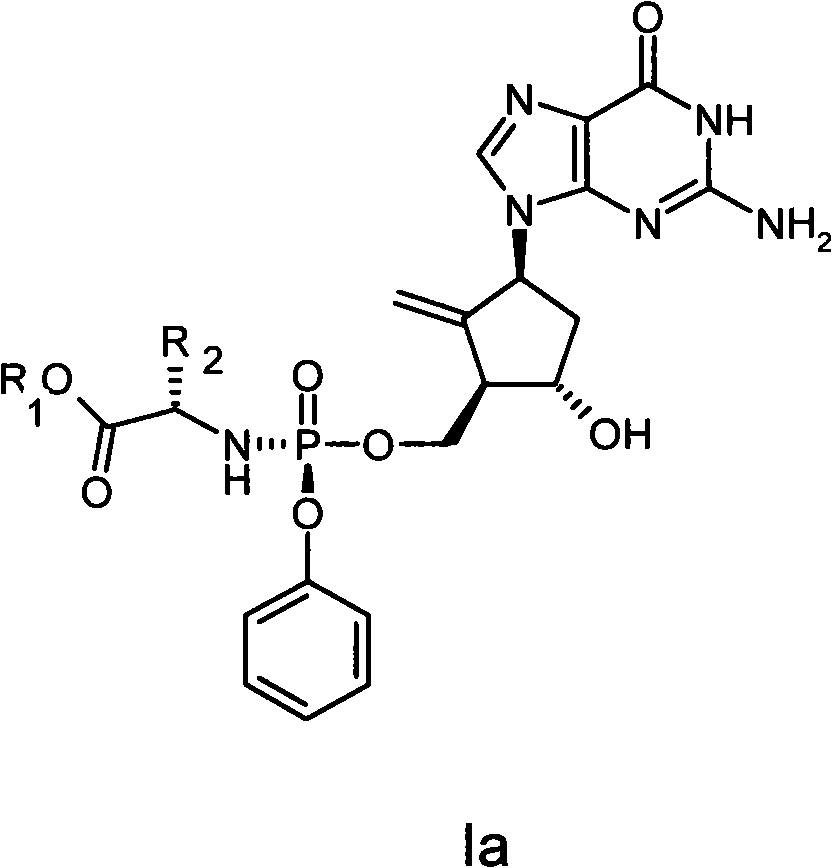
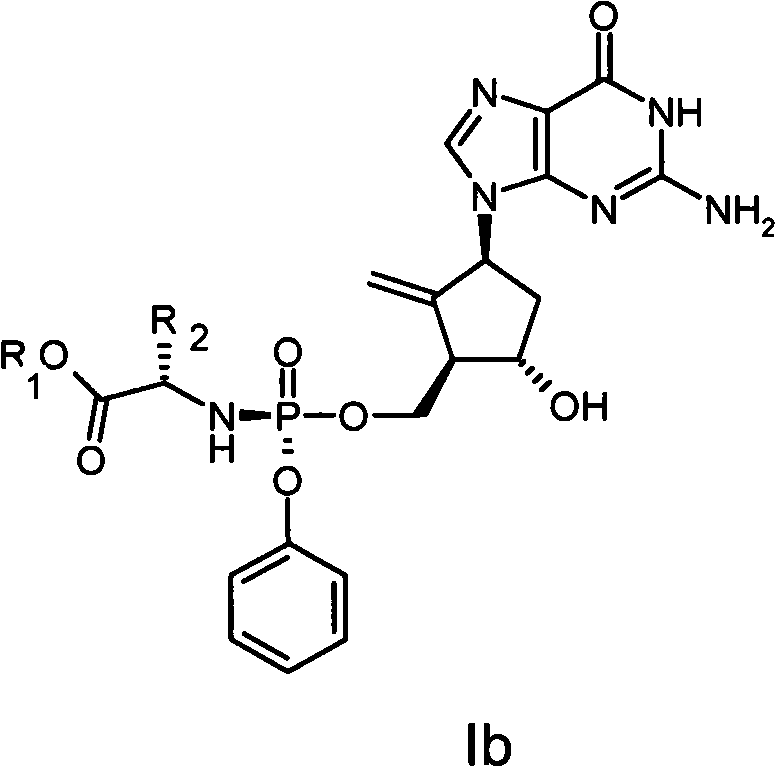
Anti-hepatitis B virus drug
An anti-hepatitis B and drug technology, applied in the field of anti-hepatitis B virus drugs, can solve the problems of poor curative effect and easy rebound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0019] Example 12-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxyl-3-((methoxycarbonylmethylamino-phenoxy-phosphoryl)-oxomethanol Base)-2-methylenecyclopentyl]-6H-purin-6-one (I-1) preparation
[0020]
[0021] Dissolve 2.1 g (0.01 mol) of phenol dichlorophosphate and 1.3 g (0.01 mol) of glycine methyl ester hydrochloride in 30 mL of anhydrous dichloromethane, and cool to -78°C. A solution of 2 g of triethylamine dissolved in 20 mL of anhydrous dichloromethane was added dropwise with stirring, and the rate of addition was controlled to maintain the reaction temperature at -78°C. After the addition was complete, the reaction temperature was gradually raised to room temperature, and stirring was continued for 1 hour. The solvent was distilled off under reduced pressure, and 30 ml of anhydrous diethyl ether was added to the residue, and filtered. The filtrate was evaporated to dryness under reduced pressure to obtain a colorless oily substance, namely phosphoramide intermediate IV...
Embodiment 2
[0024] Example 2 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxyl-3-((ethoxycarbonylmethylamino-phenoxy-phosphoryl)-oxygen Preparation of methyl)-2-methylenecyclopentyl]-6H-purin-6-one (I-2)
[0025]
[0026] Dissolve 2.1 g (0.01 mol) of phenol dichlorophosphate and 1.4 g (0.01 mol) of ethyl glycine hydrochloride in 30 mL of anhydrous dichloromethane, and cool to -78°C. A solution of 2 g of triethylamine dissolved in 20 mL of anhydrous dichloromethane was added dropwise with stirring, and the rate of addition was controlled to maintain the reaction temperature at -78°C. After the addition was complete, the reaction temperature was gradually raised to room temperature, and stirring was continued for 1 hour. The solvent was distilled off under reduced pressure, and 30 ml of anhydrous diethyl ether was added to the residue, and filtered. The filtrate was evaporated to dryness under reduced pressure to obtain a colorless oily substance, namely phosphoramide intermediate IV-2, whi...
Embodiment 32
[0028] Example 32-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxyl-3-((isopropoxycarbonylmethylamino-phenoxy-phosphoryl)-oxygen Preparation of methyl)-2-methylenecyclopentyl]-6H-purin-6-one (I-3)
[0029]
[0030] Dissolve 2.1 g (0.01 mol) of phenol dichlorophosphate and 1.53 g (0.01 mol) of glycine isopropyl hydrochloride in 30 mL of anhydrous dichloromethane, and cool to -78°C. A solution of 2 g of triethylamine dissolved in 20 mL of anhydrous dichloromethane was added dropwise with stirring, and the rate of addition was controlled to maintain the reaction temperature at -78°C. After the addition was complete, the reaction temperature was gradually raised to room temperature, and stirring was continued for 1 hour. The solvent was distilled off under reduced pressure, and 30 ml of anhydrous diethyl ether was added to the residue, and filtered. The filtrate was evaporated to dryness under reduced pressure to obtain a colorless oily substance, namely phosphoramide intermediate I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com