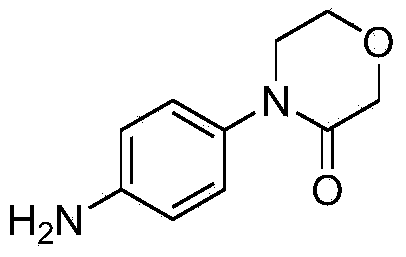
Preparation method of 4-(4-amino phenyl)-3-morpholone and intermediate of 4-(4-amino phenyl)-3-morpholone
An aminophenyl and intermediate technology, applied in the field of preparation of 4-(4-aminophenyl)-3-morpholinone, can solve the problem of high raw material prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
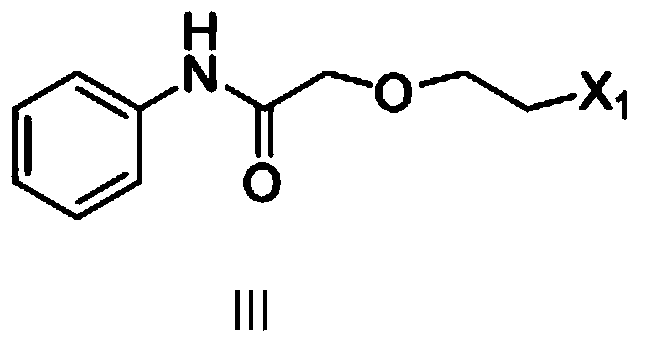
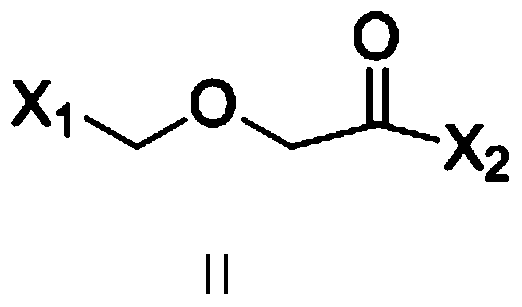
[0041] Preparation of 2-(2-chloroethoxy)-N-phenyl-acetamide
[0042] Add 93g (1mol) of aniline, 1000ml of dichloromethane, and 112g (1.1mol) of triethylamine into the reaction kettle, cool in an ice bath to 0°C, and dropwise add 157g (1mol) of 2-(2-chloroethoxy) acetyl chloride ), stirring at 25°C for 4 hours, adding 200ml of water, separating, washing with water, drying with anhydrous sodium sulfate, filtering, and recovering under reduced pressure to dryness to obtain 205g of brown oil, with a yield of 96%.
[0043] Preparation of 4-phenyl-3-morpholinone
[0044] Add 106g (0.5mol) of 2-(2-chloroethoxy)-N-phenylacetamide, 800ml of DMF, and 138g (1mol) of potassium carbonate into the reaction kettle, heat at 100°C for 8h, cool, add 4000ml of water, and filter , washed with water, and dried to obtain 69 g of a yellow-white solid, with a yield of 78%.
[0045] Preparation of 4-(4-nitrophenyl)-3-morpholinone
[0046] Add 177g (1mol) of 4-phenyl-3-morpholinone and 1200ml of ace...
Embodiment 2
[0050] Preparation of 2-(2-((phenylsulfonyl)oxy)ethoxy)-N-phenyl-acetamide
[0051] Add 93g (1mol) of aniline, 1000ml of dichloromethane, and 101g (1mol) of triethylamine into the reaction kettle, cool in an ice bath to 0°C, add 2-(2-((benzenesulfonyl)oxy)ethoxy ) Acetyl chloride 278g (1mol), after dropping, stirred at 0-5°C for 4h, added 200ml of water, separated, washed with water, dried over anhydrous sodium sulfate, filtered, recovered under reduced pressure to dryness to obtain 291g of brown oil, with a yield of 87%.
[0052] Preparation of 4-phenyl-3-morpholinone
[0053] Add 167.5g (0.5mol) of 2-(2-((phenylsulfonyl)oxy)ethoxy)-N-phenyl-acetamide, 800ml of DMF, and 138g (1mol) of potassium carbonate into the reaction kettle, heat for 100 React at ℃ for 8 hours, cool, add 4000ml of water, filter, wash with water, and dry to obtain 71g of yellow-white solid with a yield of 81%.
[0054] Preparation of 4-(4-nitrophenyl)-3-morpholinone
[0055] Add 177g (1mol) of 4-phenyl...
Embodiment 3
[0059] Preparation of 2-(2-((methylsulfonyl)oxy)ethoxy)-N-phenyl-acetamide
[0060] 93g (1mol) of aniline, 1000ml of methyl tert-butyl ether, and 101g (1mol) of triethylamine were added to the reaction kettle, cooled to 0°C in an ice bath, and 2-(2-((methylsulfonyl)oxy) was added dropwise Ethoxy) acetyl chloride 216g (1mol), after dropping, stir at 0-5°C for 4h, add 200ml of water, separate liquid, wash with water, dry with anhydrous sodium sulfate, filter, recover under reduced pressure to dryness to obtain 207g of brown oil, yield 76%.
[0061] Preparation of 4-phenyl-3-morpholinone
[0062] Add 136.5g (0.5mol) of 2-(2-((methylsulfonyl)oxy)ethoxy)-N-phenyl-acetamide, 800ml of DMF, and 138g (1mol) of potassium carbonate into the reaction kettle, heat for 100 React at ℃ for 8 hours, cool, add 4000ml of water, filter, wash with water, and dry to obtain 75g of yellow-white solid with a yield of 85%.
[0063] Preparation of 4-(4-nitrophenyl)-3-morpholinone
[0064] Add 177g (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com