Synthesis method for biphenyl compounds
A synthesis method and compound technology, which is applied in the field of synthesis of biphenyl compounds, can solve the problems of expensive, unstable ligands, and Pd cannot be recycled, and achieve the effects of low cost, simple recycling, and prevention of agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
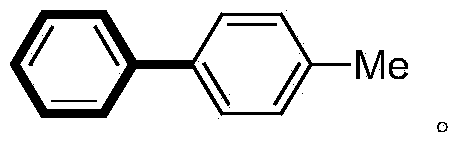
[0023] In a 100mL pressure-resistant reaction tube, add magneton, p-iodotoluene (5mmol), phenylboronic acid (7.5mmol), ZrO 2 -Pd(OAc) 2 Catalyst (280mg, containing 2.8mg palladium acetate, 0.25mol%), K 2 CO 3 (10mmol), water (5mL) and ethanol (15mL). The reaction tube was sealed with a silicone rubber gasket and heated in an oil bath at 100° C. for 0.5 hour. The reaction was complete by gas phase detection, and the catalyst was recovered by filtration. The catalyst was washed successively with water (4 mL×2), ethyl acetate (100 mL×2). After the filtrates were combined, NaOH aqueous solution was added and the aqueous layer was separated. The organic layer was washed successively with saturated NaCl aqueous solution, anhydrous NaCl 2 SO 4 dried, concentrated under reduced pressure 1 H-NMR detection is determined to be a high-purity coupling product with a yield of 97%, and the structure is as follows:
[0024]
Embodiment 2-5
[0026] In a 100mL pressure-resistant reaction tube, add magnetons, p-iodotoluene (5mmol), phenylboronic acid (7.5mmol), and all the ZrO recovered in Example 1 in sequence. 2 -Pd(OAc) 2 Catalyst, K 2 CO 3 (10mmol), water (5mL) and ethanol (15mL). The reaction tube was sealed with a silicone rubber gasket and heated in an oil bath at 100° C. for 0.5 hour. The reaction was complete by gas phase detection, and the catalyst was recovered by filtration. The catalyst was washed successively with water (4 mL×2), ethyl acetate (100 mL×2). After the filtrates were combined, NaOH aqueous solution was added and the aqueous layer was separated. The organic layer was washed successively with saturated NaCl aqueous solution, anhydrous NaCl 2 SO 4 dried, concentrated under reduced pressure 1 H-NMR detection confirmed that it was a high-purity coupling product. The recovered catalyst was subjected to repeated experiments, and the results are shown in Table 1.
[0027] Table 1: ZrO 2 ...
Embodiment 6
[0030] In a 10mL pressure-resistant reaction tube, add magneton, p-iodoanisole (1mmol), phenylboronic acid (1.5mmol), ZrO 2 -Pd(OAc) 2 Catalyst (56mg, containing 0.56mg palladium acetate, 0.25mol%), K 2 CO 3 (2mmol), water (1mL) and ethanol (3mL). The reaction tube was sealed with a silicone rubber gasket and heated in an oil bath at 100° C. for 0.5 hour. The reaction was complete by gas phase detection, and the catalyst was recovered by filtration. The catalyst was washed successively with water (2 mL×2), ethyl acetate (50 mL×2). After the filtrates were combined, NaOH aqueous solution was added and the aqueous layer was separated. The organic layer was washed successively with saturated NaCl aqueous solution, anhydrous NaCl 2 SO 4 Drying, obtaining a high-purity coupling product after concentrating under reduced pressure, the productive rate is 97%. 1 H-NMR detection, the structure is as follows:
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com