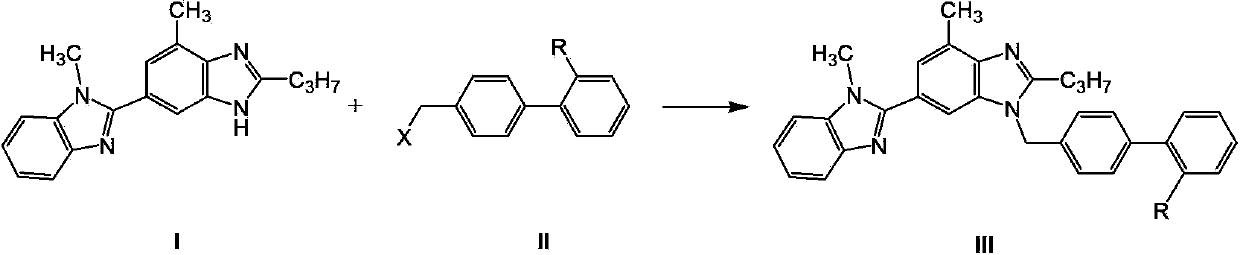
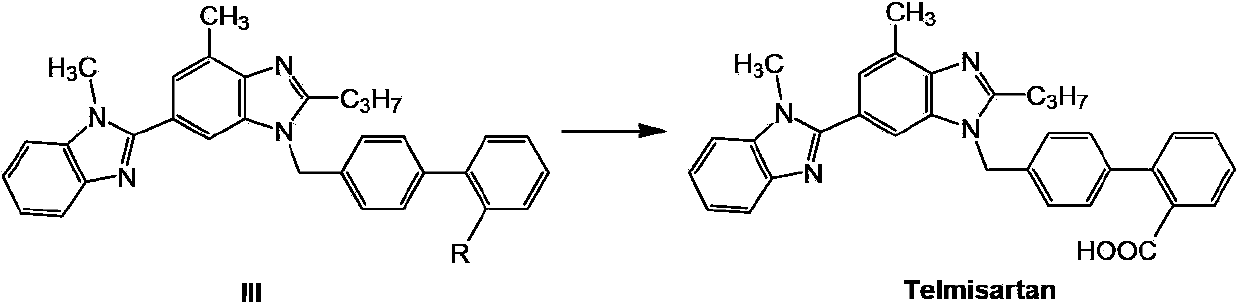
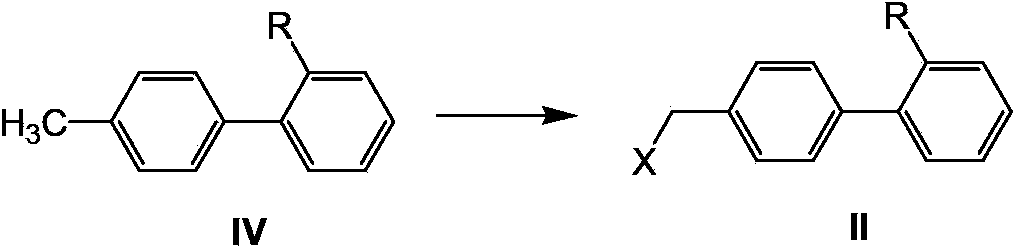
Telmisartan preparation method and intermediate of telmisartan
A kind of technology of telmisartan and compound, applied in the field of preparation of intermediate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1: Preparation of 4'-chloromethylbiphenyl-2-carboxylic acid (II, R=COOH, X=Cl)
[0105] Add 4'-methylbiphenyl 2-carboxylic acid (IV) (10g, 0.047mol), azobisisobutylcyanide (AIBN) (0.11g, 1.5mol%) into chlorobenzene (35mL), stir, Heat to 90°C, slowly add SO 2 Cl 2 (3.8mL, 0.047mol) of chlorobenzene solution (15mL), after the dropwise addition, stir for 1 hour, and TLC detects that the reaction is over; the reaction solution is naturally cooled to room temperature, and solids are precipitated, then cooled in an ice bath for 1 hour, and suction filtered , the obtained filtrate was washed with toluene (10mL×2), and dried to obtain white granular solid II (9.5g, yield 82%). 1 H NMR (300MHz, DMSO-d6): 12.79 (s, 1H, OH), 7.21-7.75 (m, 8H, ArH), 4.81 (s, 2H, CH2), MS: 246.1.
Embodiment 2
[0106] Example 2: Preparation of 4'-chloromethylbiphenyl-2-carboxylic acid (II, R=COOH, X=Cl)
[0107] Add 4'-methylbiphenyl 2-carboxylic acid (IV) (10g, 0.047mol), azobisisobutylcyanide (AIBN) (0.11g, 1.5mol%) into chlorobenzene (70mL), stir, Heat to 80°C, add trichloroisocyanuric acid (10.9g, 0.047mol), stir for 12 hours, and filter out the insoluble matter; naturally cool the reaction solution to room temperature, and solids are precipitated, and the obtained filtrate is washed with toluene ( 10mL×2), dried to obtain white granular solid II (6g, yield 52%).
Embodiment 3
[0108] Example 3: Preparation of 4'-chloromethylbiphenyl-2-carboxylic acid (II, R=COOH, X=Cl)
[0109] Dissolve 4'-methylbiphenyl 2-carboxylic acid (IV) (10g, 0.047mol) in dichloromethane (150mL), add benzoyl peroxide (0.23g, 2mol%), add 1.1 equivalent of chlorine, The reaction was stirred for 12 hours; saturated aqueous sodium bicarbonate solution was added to the reaction solution for extraction, the organic layer was separated, and the solvent was evaporated to dryness to obtain off-white solid II (6 g, yield 52%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com