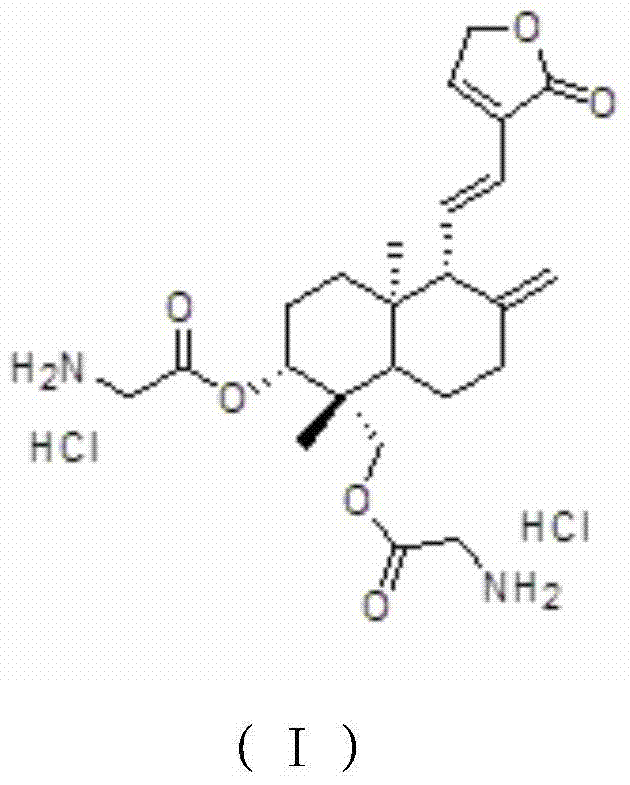
Novel application of dehydroandrographolide derivative
A technology for dehydrating andrographis paniculata and derivatives, applied in the field of medicine, can solve the problems such as the application of andrographolide and its derivatives for senile dementia, which has not yet been seen, and achieve the effect of good development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
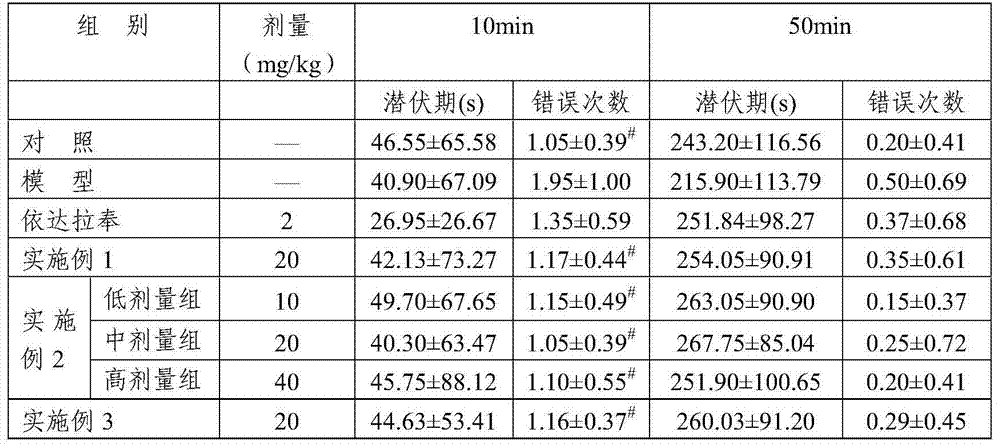
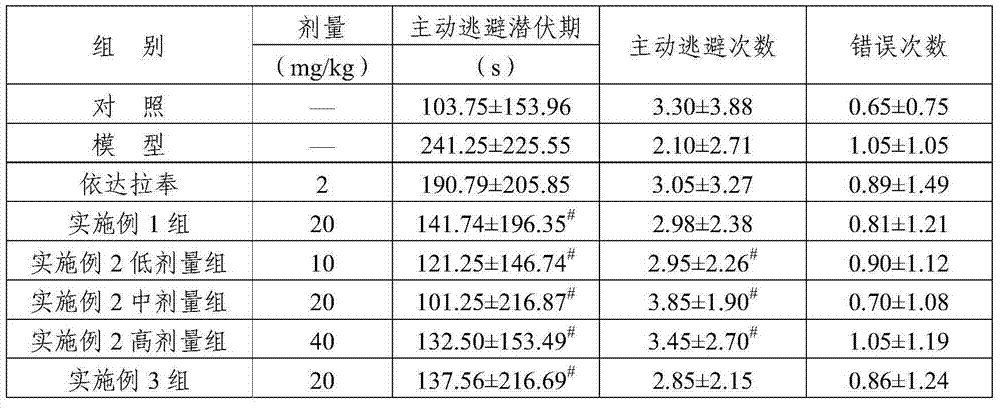
Examples
Embodiment 1
[0026] Embodiment 1: the preparation method of dehydroandrographolide glycine half ester hydrochloride
[0027] 1) Dissolve 6.64g dehydroandrographolide, 10.5g N-benzyloxycarbonyl-glycine in 100ml N,N-dimethylformamide, then add 10.3g dicyclohexylcarbodiimide and 0.24g4 -Dimethylaminopyridine, warmed to room temperature and stirred for 8h, filtered, and the filtrate was evaporated to dryness under reduced pressure; the residue was separated by silica gel column chromatography, and eluted with a mixed solvent of ethyl acetate:petroleum ether (1:3 by volume) , collected the target components, and concentrated to dryness under reduced pressure to obtain 8.8 g of 3,19-0-bis-(N-benzyloxycarbonylglycyl)-dehydroandrographolide;
[0028] 2) Dissolve 8.8 g of 3,19-0-bis-(N-benzyloxycarbonylglycyl)-dehydroandrographolide in 100 ml of ethanol, add 1.2 g of 10% palladium carbon, and store at room temperature under a hydrogen atmosphere of 1 atmosphere After stirring for 3 hours, the pall...
Embodiment 2
[0029] Embodiment 2: Dehydroandrographolide derivative composition freeze-dried powder injection
[0030] 1) Dissolve 0.3 g of disodium edetate in 500 ml of water for injection, add 40 g of dehydrated andrographolide glycine half ester hydrochloride prepared in Example 1, stir to dissolve, and obtain a solution;
[0031] 2) Add 30g of mannitol to the solution to dissolve it, adjust the pH value to 7.5, add water for injection to 1000ml, filter the resulting liquid deeply, divide the liquid into vials, 1ml per bottle, and transfer to the freezer in the dryer;
[0032] 3) Freeze-dry the liquid medicine, press the plug, and seal it to obtain the product.
Embodiment 3
[0033] Embodiment 3: dehydroandrographolide derivative aqueous injection
[0034] 1) Dissolve 0.1 g of calcium sodium edetate in 400 ml of water for injection, add 10 g of dehydrated andrographolide glycine half ester hydrochloride prepared in Example 1, stir to dissolve, and obtain a solution;
[0035] 2) Add 40g of fructose to the solution to dissolve it, adjust the pH value to 6.8, add water for injection to 1000ml, filter the resulting liquid in depth, wait for the liquid to cool to 30°C, fill it, 2ml per bottle, and extinguish at 115°C Bacteria for 40 minutes, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com