SSR markers for plants and uses thereof
A technology of markers and plants, applied to the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the problems of undetected polymorphisms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
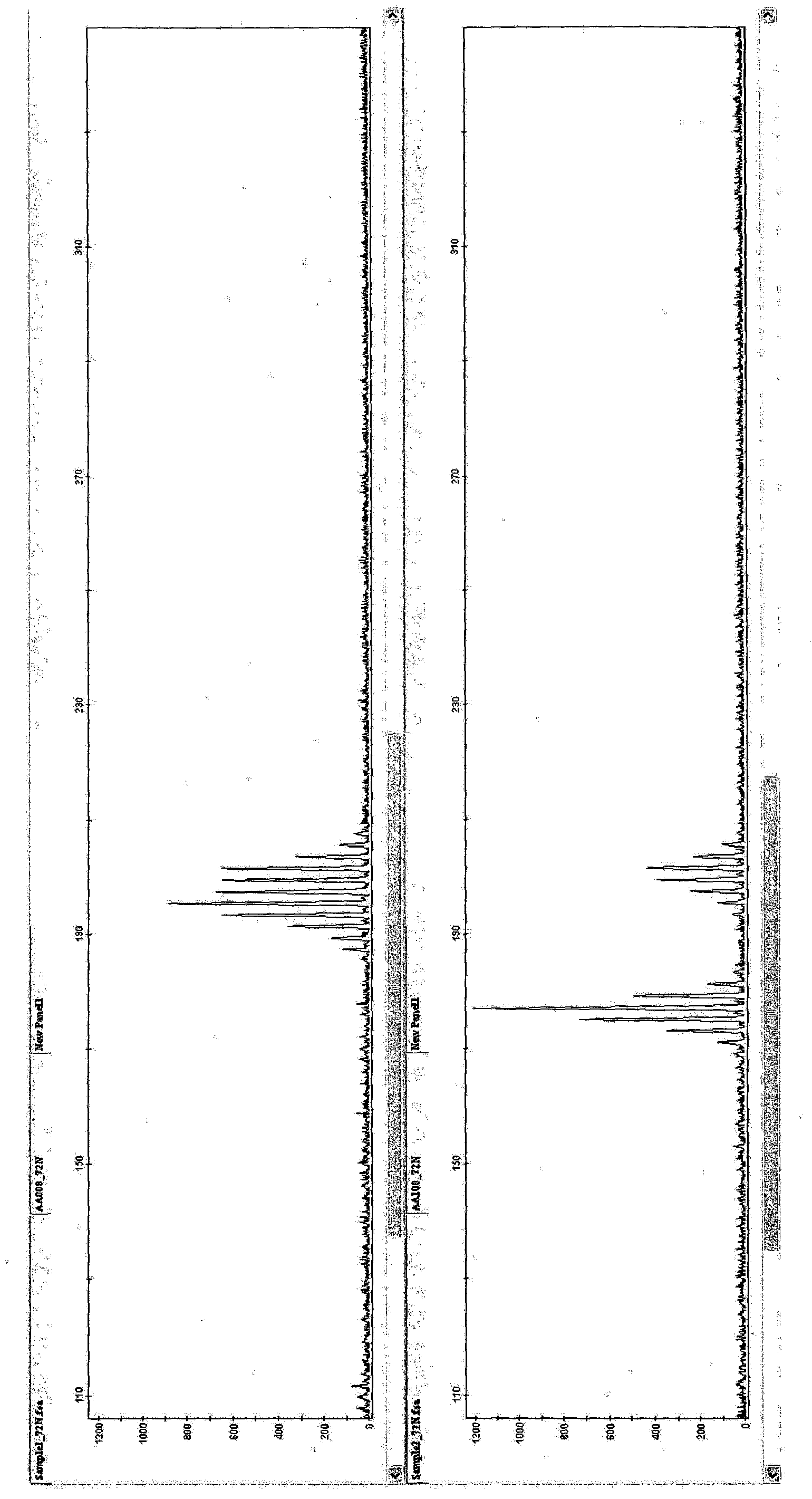
[0078] [Example 1: PCR amplification of SSR markers using oligonucleotide primers in Table 1 and detection by capillary electrophoresis]
[0079] 10 μl of reaction mixture for SSR marker amplification consisted of: 2 mM MgCl 2 , 1×PCR buffer, 0.2 mM of dNTP mix, 250 nM of each primer of the primer pair, 1 U of Taq polymerase and 20 ng of DNA. Cycle conditions are denaturation at 94°C, 5min, 5 cycles: 94°C, 30s; 62°C, 30s (decrease 2°C to 52°C); 25 cycles: 94°C, 30s; 52°C, 30s, 72°C, 30s; Then at 72°C for 7min, and kept at 10°C. in 96-well Amplification is performed in the PCR system 9700. Other reaction mixtures, cycling conditions and thermal cyclers with heated lids can also be used.
[0080] For capillary electrophoresis, fluorescently labeled primers are used to amplify the products. Amplified products were analyzed by capillary electrophoresis. Dimensions are determined using standards. Samples and size standards (GeneScan600LIZ) were heated and loaded into an App...
Embodiment 2
[0082] [Example 2: Polyacrylamide gel electrophoresis (PAGE)]
[0083] PCR was performed using the same conditions as in Example 1. Amplification products were separated using polyacrylamide gel electrophoresis.
[0084] The amplified PCR products were heated with conventional formamide loading dye and 10 μL of each sample was separated in a 7% polyacrylamide gel (1 mm thick and 25 cm long) at 450 V for 6 h. Table 3 shows an example of polyacrylamide gel formulation.
[0085] Table 3: Polyacrylamide Formulations
[0086] 17.5ml
Concentrated stock acrylamide solution (19g acrylamide, 1g bisacrylamide in 100mL water)
10ml
5×TBE (1×TBE=0.09M Tris-boric acid, 0.002M EDTA)
22.5ml
water
220μl
10% ammonium persulfate (10%APS)
20 μL
TEMED
[0087] Add 10% APS and TEMED to aggregate.
[0088] After electrophoresis, the separated amplification products were visualized by silver staining or ethidium bromide staining. D...
Embodiment 3
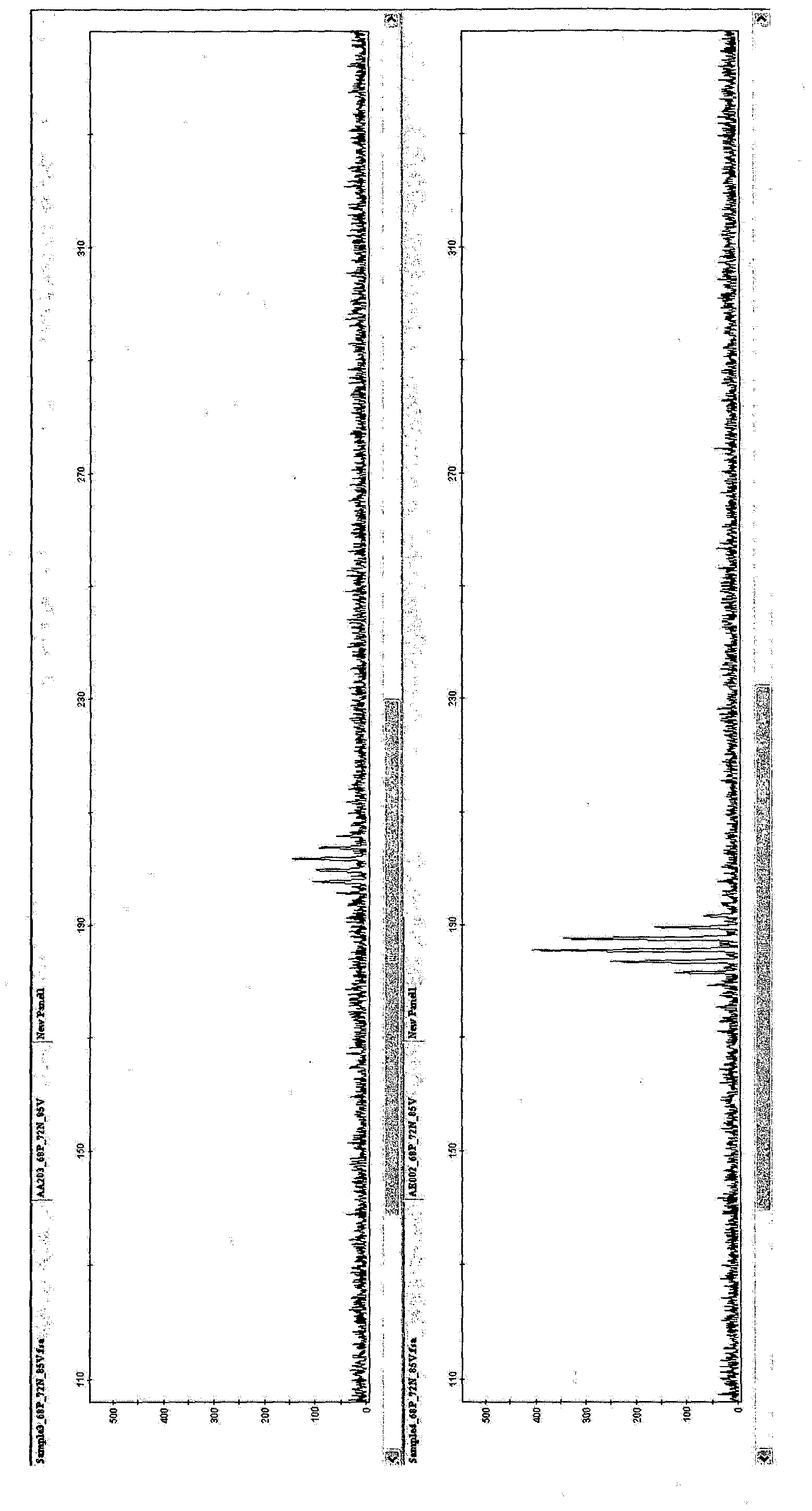
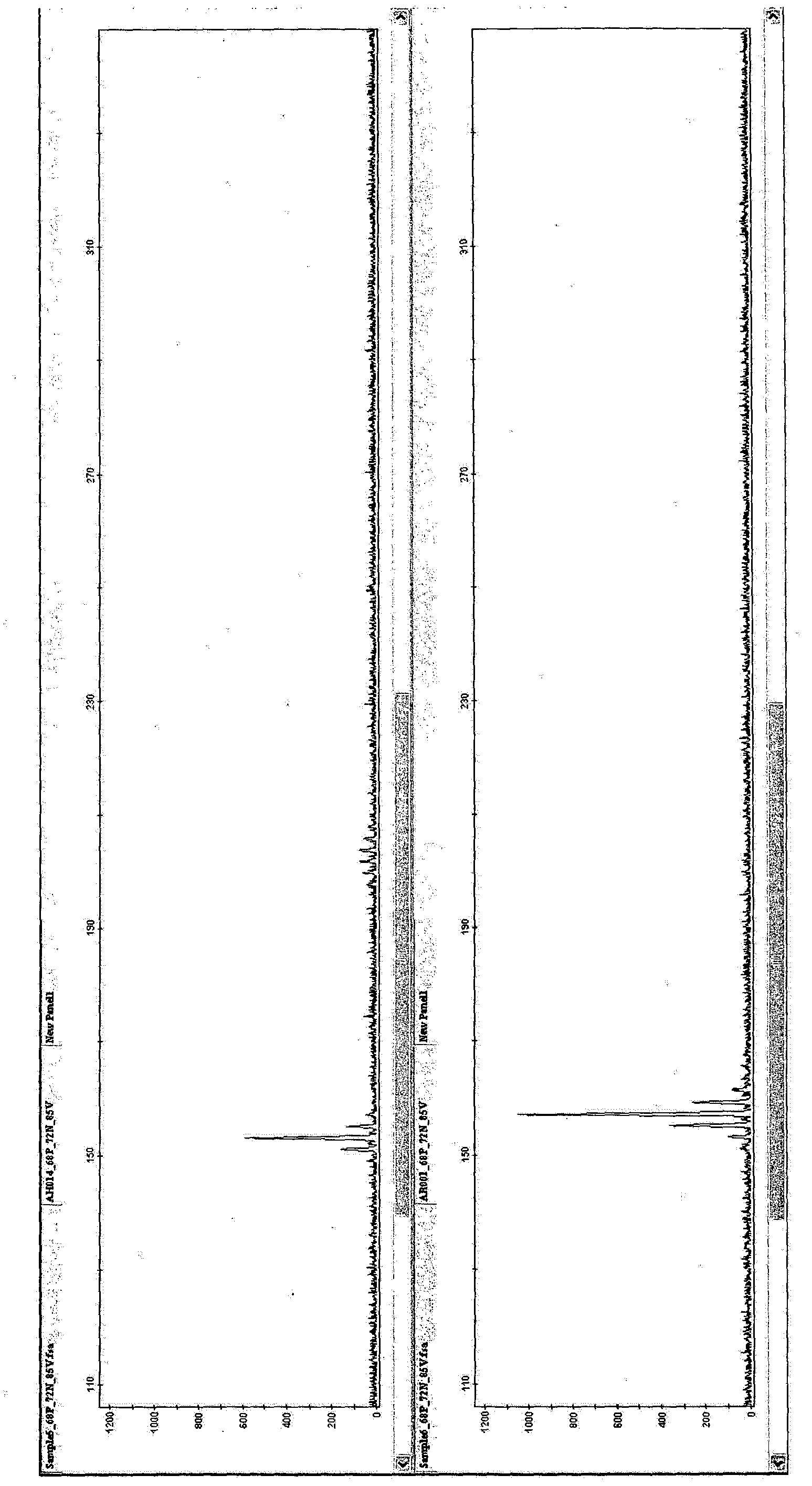
[0089] [Example 3: Cluster Analysis]
[0090] Genotyping of 927 samples was performed using the 10 SSR markers of the present invention. For each marker, PCR was performed using the PCR mix shown in Table 4 in a volume of 10 μl.
[0091] Table 4: PCR mix
[0092] PCR mix
10 μl PCR
h 2 o
1.69
10×PCR buffer
1.00
50mM MgCl 2
0.40
10mM dNTPs
0.20
Primer mix F:R (25μM:50μM)
0.05
M13-labeled fluorescent forward primer (2.5 μM)*
0.50
Taq5u / μl
0.16
DNA (3ng / μl)
6.00
total capacity
10.00
[0093] * Indicates M13-labeled fluorescent forward primers (labeled fluorescent forward primers are also shown in Table 1 with *).
[0094] Samples and size standards (GeneScan600LIZ) were heated and loaded into an Applied Biosystems ABI3730xl Genome Analyzer (a fluorescence-based sequencer) according to the manufacturer's instructions. Scoring was performed with Applied B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com