Cefazolin derivative and its preparation method, oral antibiotic preparation
A technology for cefazolin and cefazolin acid, which is applied in the fields of cefazolin derivatives and their preparation, and oral antibiotic preparations, and achieves the effects of easily controllable reaction conditions, reduced incidence and good drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Correspondingly, the present invention also provides a kind of synthetic method of above-mentioned cefazolin derivative, and this method comprises the steps:
[0025] S01. Prepare the mixed solution of the low-temperature reactant of cefazolin core and cefazolin acid: dissolve the cefazolin core and cefazolin acid in the reaction solvent and lower the temperature to below 5° C., then add a condensing agent to obtain Low temperature reactant mixed solution;
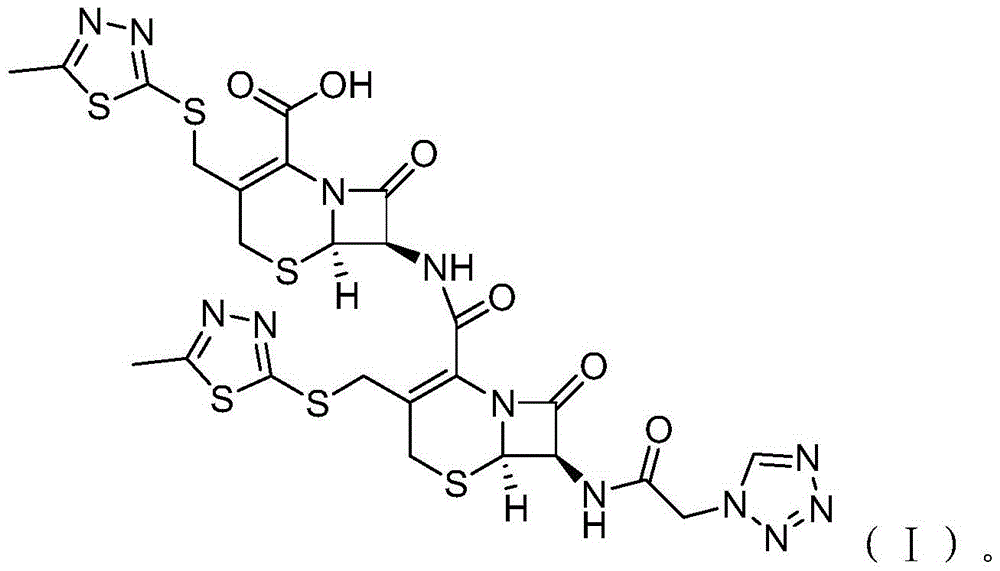
[0026] S02. Heating the low-temperature reactant solution for condensation reaction: heating the low-temperature reactant mixed solution prepared in step S01 to 20-30°C for condensation reaction, and after the reaction is completed, ice water is precipitated, solid-liquid separation, and purification treatment , to obtain the cefazolin derivative of the following molecular structural formula (I):
[0027]
[0028] Specifically, in the above-mentioned step S01, the purpose of dissolving the cefazolin mother nucle...
Embodiment 1
[0047] A kind of cefazolin derivative and its synthetic method
[0048] Cefazolin nuclei (3.4g) and cefazolin acid (4.5g) were dissolved in tetramethylguanidine (30ml), and the temperature was lowered to 0°C, and slowly added into the reaction solution and 1-ethyl-(3-di Methylaminopropyl) carbodiimide hydrochloride (2.2g), fully stirred to dissolve, the temperature was raised to 25°C, and the reaction was stirred for 12 hours; the reaction was stopped, and the reaction solution was poured into 1000ml of ice water, filtered to obtain a large amount of White solid, the filter cake was washed with sodium carbonate solution of pH7.5; the filter cake was dissolved in chloroform, separated by silica chromatography column to obtain 5.8 grams of cefazolin derivatives (formula I), with a purity of 99.3%. Its mass yield is 171%.
[0049] The cefazolin derivative prepared by the embodiment of the present invention 1 is carried out proton nuclear magnetic resonance spectrum analysis and ...
Embodiment 2
[0053] Cefazolin core (3.4g) and cefazolin acid (5.0g) were dissolved in tetramethylguanidine (100ml), and the temperature was lowered to 2°C, and slowly added to the reaction solution and 1-ethyl-(3-di Methylaminopropyl) carbodiimide hydrochloride (2.6g), fully stirred to dissolve, the temperature was raised to 22°C, and the reaction was stirred for 12 hours; the reaction was stopped, and the reaction liquid was poured into 1000ml of ice water, filtered to obtain a large amount of White solid, the filter cake was washed with sodium carbonate solution of pH7.8; the filter cake was dissolved in chloroform, separated by silica chromatography column to obtain 5.2 grams of cefazolin derivatives (formula I), with a purity of 99.5%. Its mass yield is 153%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com