Cefazolin axetil and its preparation method, oral antibiotic preparation
A technology of cefazolin proxetil and cefazolin, which is applied in the field of antibiotic drugs to achieve the effects of reducing the incidence rate, improving drug efficacy, and changing water-soluble properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] Correspondingly, the present invention also provides a kind of synthetic method of above-mentioned cefazolin axetil, this method comprises the steps:
[0024] S01. Preparation of cefazolin acid-base salt: reacting cefazolin acid and acetic acid-base salt at 0-5°C in a reaction solvent to obtain cefazolin acid-base salt;
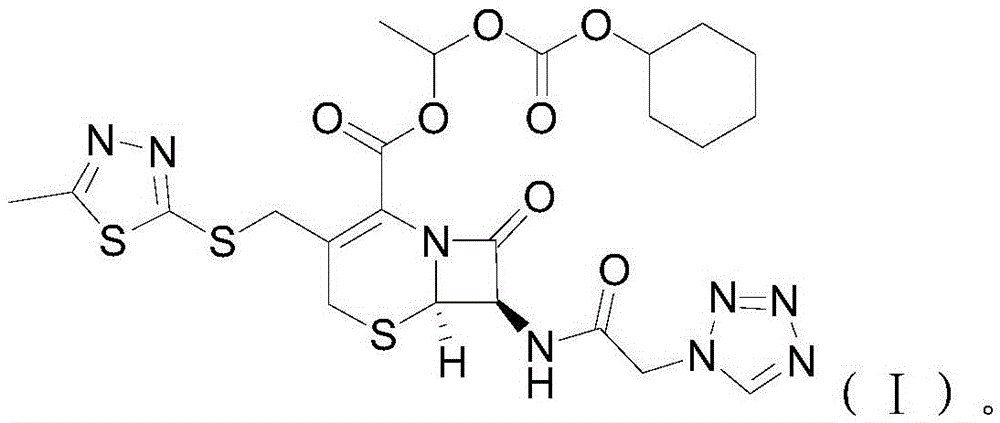
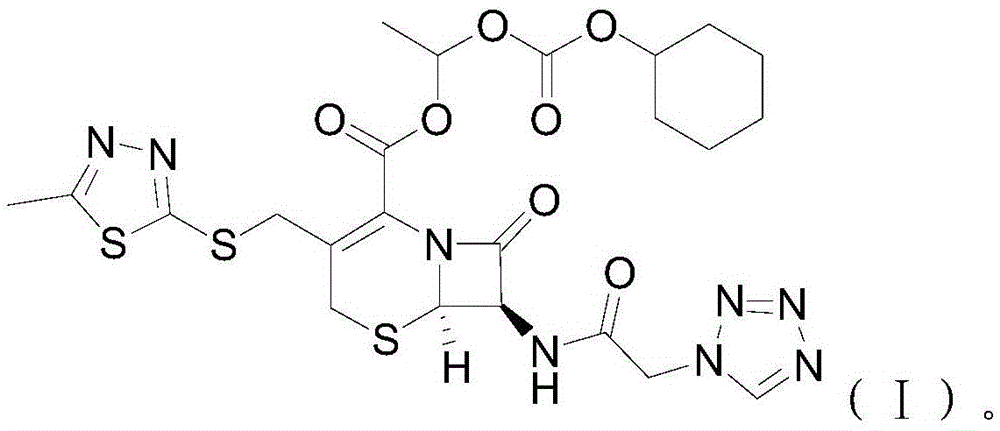
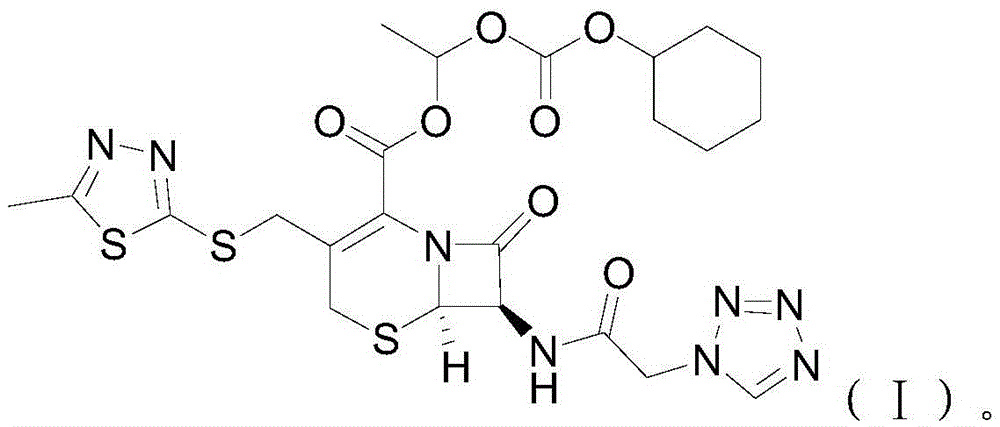
[0025] S02. Esterification of cefazolin acid-base salt and 1-iodoethyl cyclohexyl carbonate: the cefazolin acid-base salt prepared in step S01 and the 1-iodoethyl carbonate prepared in step S02 Cyclohexyl ester is esterified in an anhydrous solvent containing potassium carbonate and potassium dihydrogen phosphate at -10~-5°C. After the reaction is completed, add ice water and carry out crystal growth treatment, and then carry out solid-liquid separation, Purification treatment, obtains the described cefazolin axetil of following molecular structural formula (I):
[0026]
[0027] Specifically, the chemical reaction formula between the cefazolin aci...
Embodiment 1
[0056] A kind of cefazolin proxetil and its synthetic method
[0057] S11. Preparation of Cefazolin Potassium:
[0058] Under anhydrous conditions at 0°C, 45.4 g (0.1 mol) of cefazolin acid was dissolved in 50 ml of methanol and 100 ml of dry DMF. Add 10.8 grams of potassium acetate in batches under temperature control (0°C), react under temperature control for 1 hour, and filter; control the temperature of the filtrate at 20°C, add 800 ml of isopropanol dropwise, and finish dripping for 35 minutes. The cake was washed with 200ml of isopropanol and dried under reduced pressure with phosphorus pentoxide to obtain 40.1g of off-white to pale yellow cefotiam potassium salt.
[0059] S12. Preparation of 1-iodoethyl cyclohexyl carbonate:
[0060] Under the condition of anhydrous 40°C, add sodium iodide (75g, 0.5mol) and powdered anhydrous calcium chloride (16.6g, 0.075mol) into 200ml of anhydrous acetonitrile with stirring in the dark, stir for 10min, add carbonic acid-1 -Chloroe...
Embodiment 2
[0067] A kind of cefazolin proxetil and its synthetic method
[0068] S11. Preparation of Cefazolin Potassium:
[0069] 45.4 g (0.1 mol) of cefazolin acid was dissolved in 50 ml of ethanol and 100 ml of anhydrous DMA under anhydrous conditions at 5°C. Add 10.8 g of potassium acetate in batches under temperature control (5°C), react under temperature control for 1 hour, and filter; control the temperature of the filtrate at 20°C, add 800 ml of isopropanol dropwise, finish dripping for 35 minutes, grow crystals for 1.5 hours, and then filter. The cake was washed with 200ml of isopropanol and dried under reduced pressure with phosphorus pentoxide to obtain 38.2g of off-white to light yellow cefotiam potassium salt.
[0070] S12. Preparation of 1-iodoethyl cyclohexyl carbonate:
[0071] Under the condition of anhydrous 43°C, add sodium iodide (75g, 0.75mol) and powdered anhydrous calcium chloride (16.6g, 0.075mol) into 200ml of anhydrous acetonitrile with stirring in the dark, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com