Crocetin amides derivative and preparation method and application thereof
A technology of saffron acid amide and saffron acid, which is applied to a class of saffron acid amide derivatives and the fields of their preparation and use, can solve the problems of limited development and utilization, difficulty in large-scale cultivation of saffron, and high cost of extraction and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
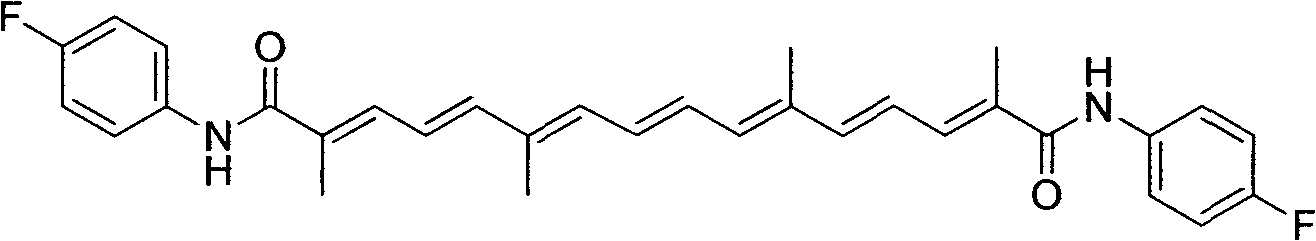
[0018] Embodiment 1: (2E, 4E, 6E, 8E, 10E, 12E, 14E)-N 1 , N 16 - Preparation of bis(4-fluorophenyl)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptanamide (compound 1)
[0019]
[0020] Add 1mmol crocetin to a 50mL round-bottomed flask and dissolve with 10mL organic solvent DMF or DMSO; add 2.2mmol oxalyl chloride and 2.1mmol triethylamine dropwise to the saffron solution at 0°C, and stir magnetically for 4h; Add 2.1 mmol of substituent aniline to the solution obtained in step 1 at low temperature, and stir for 8 hours with magnetic force; after the reaction, pour the reaction solution into water, extract with ethyl acetate, wash with saturated saline, and anhydrous sodium sulfate Dry, remove ethyl acetate by distillation under reduced pressure, and recrystallize with a mixed solution of ethanol and acetone (volume ratio 5:1) to obtain the final product. Brick red powder, yield 54%.m.p.254-255℃; 1 H NMR (300MHz, DMSO-d 6 )δ: 1.90(s, 6H), 2.01(s, 6H), 6.35-6.37(d, J...
Embodiment 2
[0021] Embodiment two: (2E, 4E, 6E, 8E, 10E, 12E, 14E)-N 1 , N 16 - Preparation of bis(4-chlorophenyl)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptanamide (compound 2)
[0022]
[0023] The preparation method is the same as in Example 1. Substituting 4-chloroaniline for 4-fluoroaniline, the title compound was obtained. Red brick-colored powder, yield 45%, m.p.265-266°C; 1 H NMR (300MHz, DMSO-d 6 )δ: 1.92(s, 6H), 1.99(s, 6H), 6.33-6.36(d, J=2.51Hz, 2H), 6.54-6.55(dd, J=5.64Hz, 2H), 6.57-6.60(d , J=7.17Hz, 2H), 6.86-6.72(dd, J=5.45Hz, 2H), 7.17-7.19(d, J=4.69Hz, 4H), 7.22-7.25(d, J=6.26Hz, 2H) , 7.86-7.89 (d, J=3.42Hz, 4H), 10.11 (s, 2H). MS (ESI): 547.18 (C 32 h 33 Cl 2 N 2 o 2 ,[M+H] + ).Anal.Calcd for C 32 h 32 Cl 2 N 2 o 2 : C, 70.20; H, 5.89; N, 5.12%. Found: C, 70.06; H, 5.91; N, 5.12%.
Embodiment 3
[0024] Embodiment three: (2E, 4E, 6E, 8E, 10E, 12E, 14E)-N 1 , N 16 - Preparation of bis(4-bromophenyl)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptanamide (compound 3)
[0025]
[0026] The preparation method is the same as in Example 1. Substituting 4-bromoaniline for 4-fluoroaniline, the title compound was obtained. Red brick-colored powder, yield 48%, m.p.291-292°C; 1 H NMR (300MHz, DMSO-d 6 )δ: 1.90(s, 6H), 2.02(s, 6H), 6.31-6.34(d, J=3.01Hz, 2H), 6.55-6.58(dd, J=5.61Hz, 2H), 6.57-6.59(d , J=6.21Hz, 2H), 6.85-6.89(dd, J=5.43Hz, 2H), 7.17-7.20(d, J=4.79Hz, 4H), 7.22-7.25(d, J=6.21Hz, 2H) , 7.83-7.85 (d, J=3.46Hz, 4H), 10.14 (s, 2H). MS (ESI): 635.08 (C 32 h 33 Br 2 N 2 o 2 ,[M+H] + ).Anal.Calcd for C 32 h 32 Br 2 N 2 o 2 : C, 60.39; H, 5.07; N, 4.40%. Found: C, 60.26; H, 5.08; N, 4.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com