Synthesis of benzsulfamide HDAC (Histone Deacetylase) inhibitor and application of benzsulfamide HDAC inhibitor in resisting tumor
A technology of p-toluenesulfonamide and a synthesis method, which is applied in the field of pharmaceutical synthesis and can solve the problems of not finding benzenesulfonamide compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
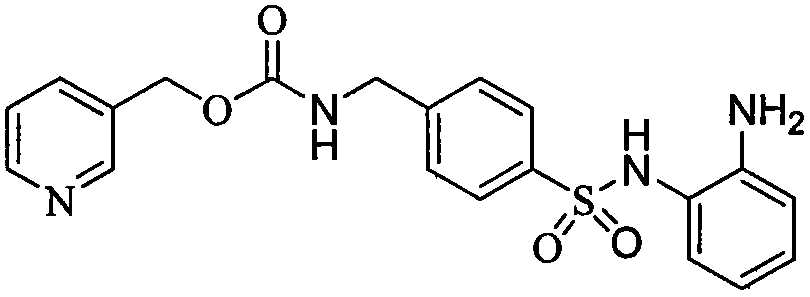
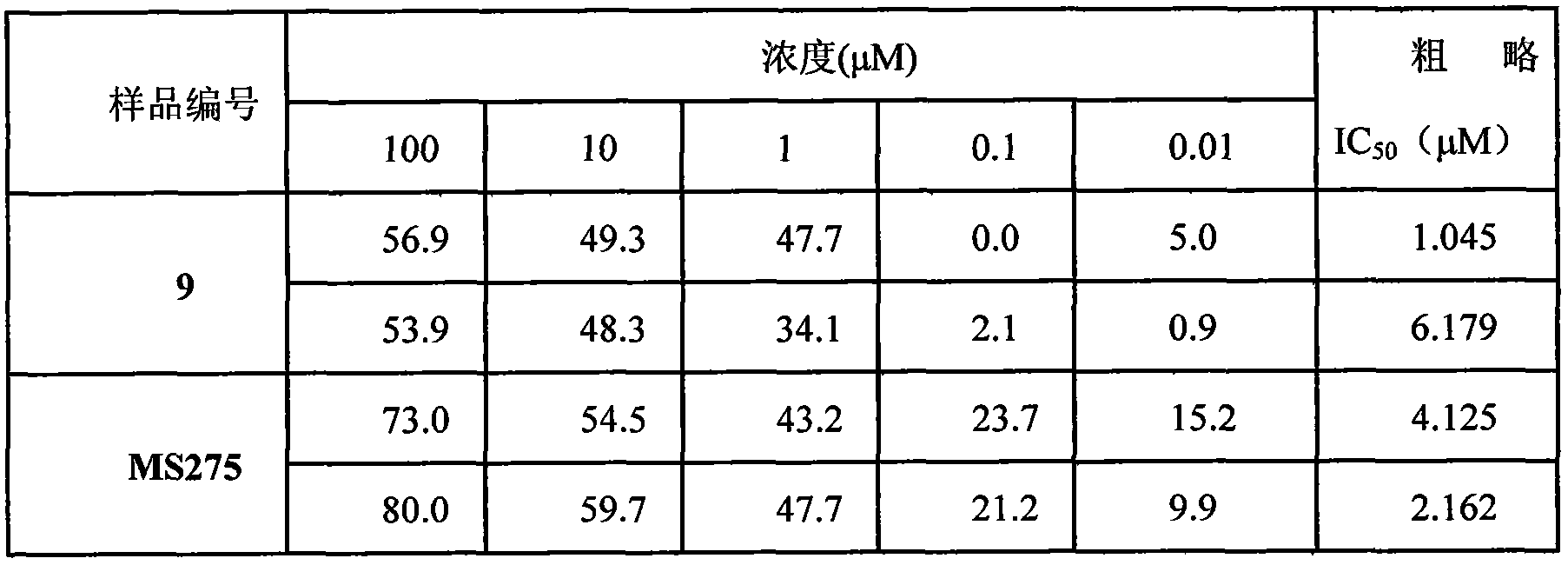
[0025] The present invention will be described in detail below with reference to the accompanying drawings and taking the preparation and anti-tumor application of benzenesulfonamide compounds as specific examples.
[0026] (1) Preparation of benzenesulfonamide compounds
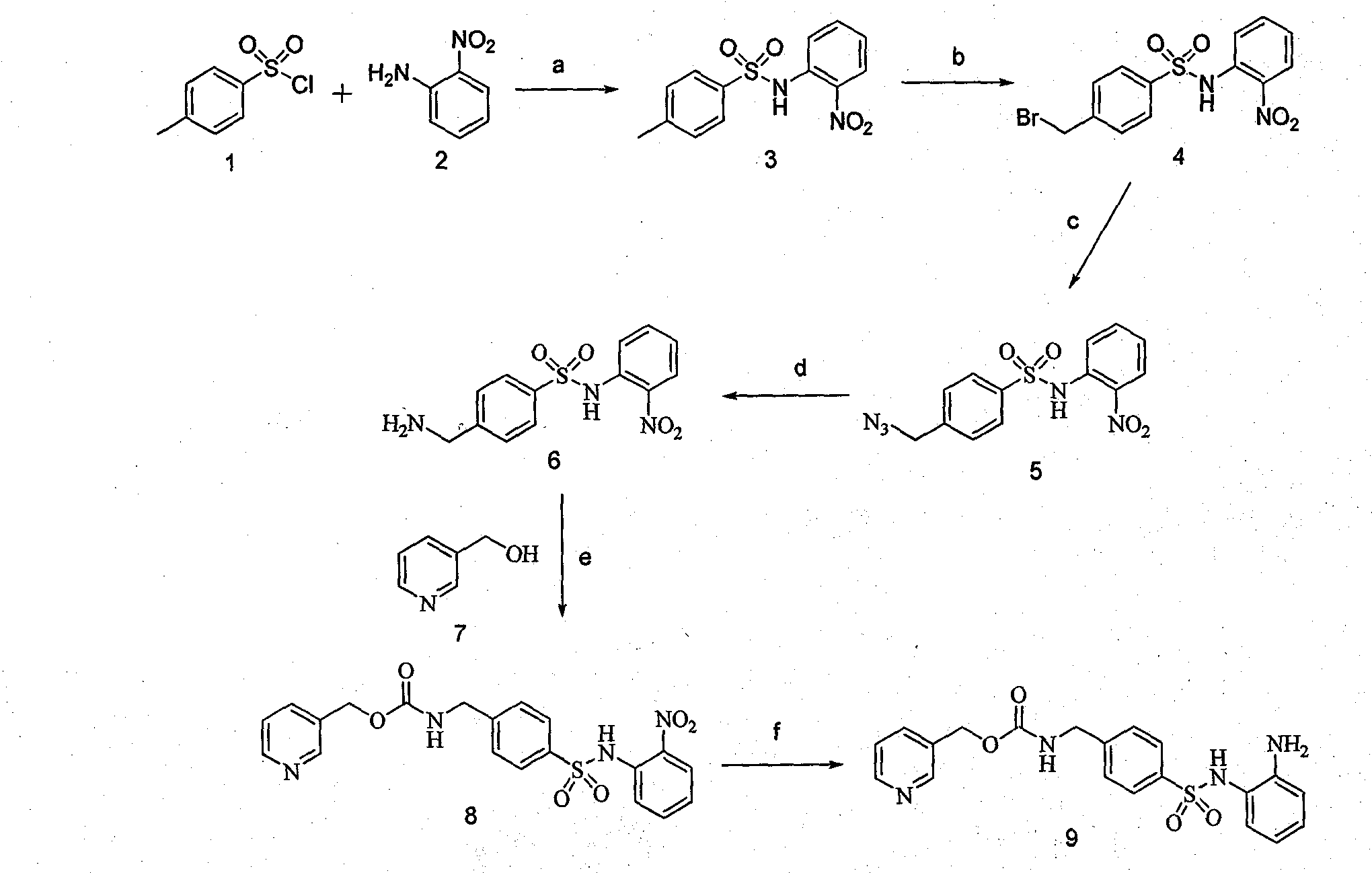
[0027] Such as figure 1 As shown, the reagents and conditions in the synthetic reaction of benzenesulfonamide HDACs inhibitors are: (a) pyridine, 125°C, 6h, 53%; (b) AIBN, NBS, CCl 4 , reflux, 3h, 61%; (c) NaN 3 , DMF, room temperature, 24h, 86%; (d) Triphenyl phosphine, THF, H 2 O, 60°C (, 12h, 52%; (e) CDI, THF, DBU, Et 3 N, 45%; (f) SNCl 2 2H 2 O, reflux, 12h, 61%.
[0028] Synthesis of compound 3:
[0029] Dissolve 1g (7.24mmol) of o-nitroaniline in 40ml of dry pyridine solution, slowly add 1.38g (7.24mmol) of p-toluenesulfonyl chloride, and react at 125°C for 6h. After the reaction is completed, pour the reaction solution into 300ml of ice water. At this time, a brown solid gradually precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com