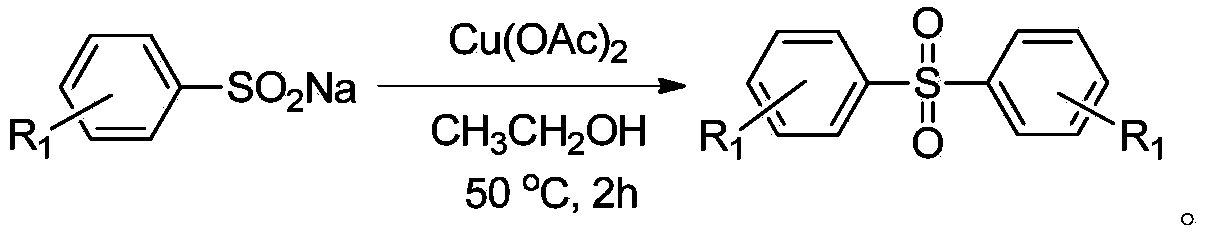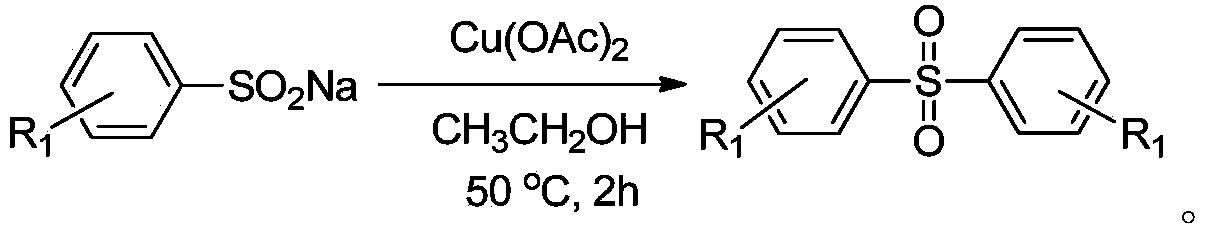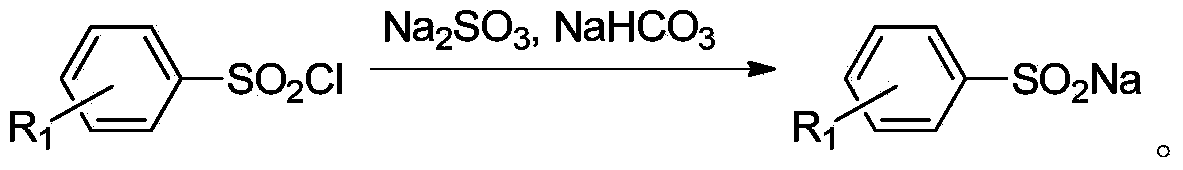Method for preparing diaryl sulfone
A technology of diaryl sulfone and aryl sulfinate, applied to the preparation of organic compounds, chemical instruments and methods, formation/introduction of sulfonyl/sulfinyl groups, etc., to achieve mild reaction conditions and high reaction yield , easy to remove effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 1 mmol of sodium p-tolylsulfinate and 0.5 mmol of copper chloride into a reaction tube filled with 2 ml of ethanol, heat to 50 degrees Celsius, and magnetically stir for 2 hours. After the reaction was completed, the solvent was spin-dried after cooling down to room temperature, and recrystallized to obtain the product with a yield of 96%.
[0036] Product Confirmation:
[0037]
[0038] 1 HNMR (400MHz, CDCl 3 ):δ7.81(d,J=7.6Hz,4H),7.20(d,J=7.6Hz,4H),2.39(s,6H); 13 C NMR (100MHz, CDCl 3 ): δ140.2, 139.9, 130.1, 129.8, 21.1; HRMS calcd for C 14 h 14 o 2 S:246.0715,found:246.0717.
Embodiment 2
[0040] Add 1 mmol of sodium p-dimethylaminophenyl sulfinate and 0.5 mmol of copper chloride into a reaction tube filled with 2 ml of ethanol, heat to 50 degrees Celsius, and stir magnetically for 2 hours. After the reaction was completed, the solvent was spin-dried after cooling down to room temperature, and the product was obtained by recrystallization with a yield of 90%.
[0041] Product Confirmation:
[0042]
[0043] 1 H NMR (400MHz, CDCl 3 ):δ7.83(d,J=8.8Hz,4H),7.25(d,J=8.8Hz,4H),3.09(s,12H); 13 C NMR (100MHz, CDCl 3 ): δ178.1, 159.1, 129.8, 123.0, 47.5. HRMS calcd for C 16 h 20 N 2 o 2 S:304.1245,found:304.1247.
Embodiment 3
[0045] Add 1 mmol of sodium p-cyanophenylsulfinate and 0.5 mmol of copper chloride into a reaction tube filled with 2 ml of ethanol, heat to 50 degrees Celsius, and stir magnetically for 2 hours. After the reaction was completed, the solvent was spin-dried after cooling down to room temperature, and the product was obtained by recrystallization with a yield of 93%.
[0046] Product Confirmation:
[0047]
[0048] 1 H NMR (400MHz, CDCl 3 ,TMS)δ7.58(d,J=7.2Hz,1H),7.51-7.54(m,3H),7.39-7.43(m,3H),7.30-7.37(m,2H).HRMS(EI)Calcd for C 12 h 9 Br(M + )231.9888, Found231.9887.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com