Method of synthesizing diphenyl-methane derivatives by using benzyl oxides as raw material
A technology for diphenylmethane and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, etc., can solve the problem of residual metal ions in products, strong corrosion of equipment, and increase in production costs. and other problems, to achieve the effect of good product quality, short response time and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
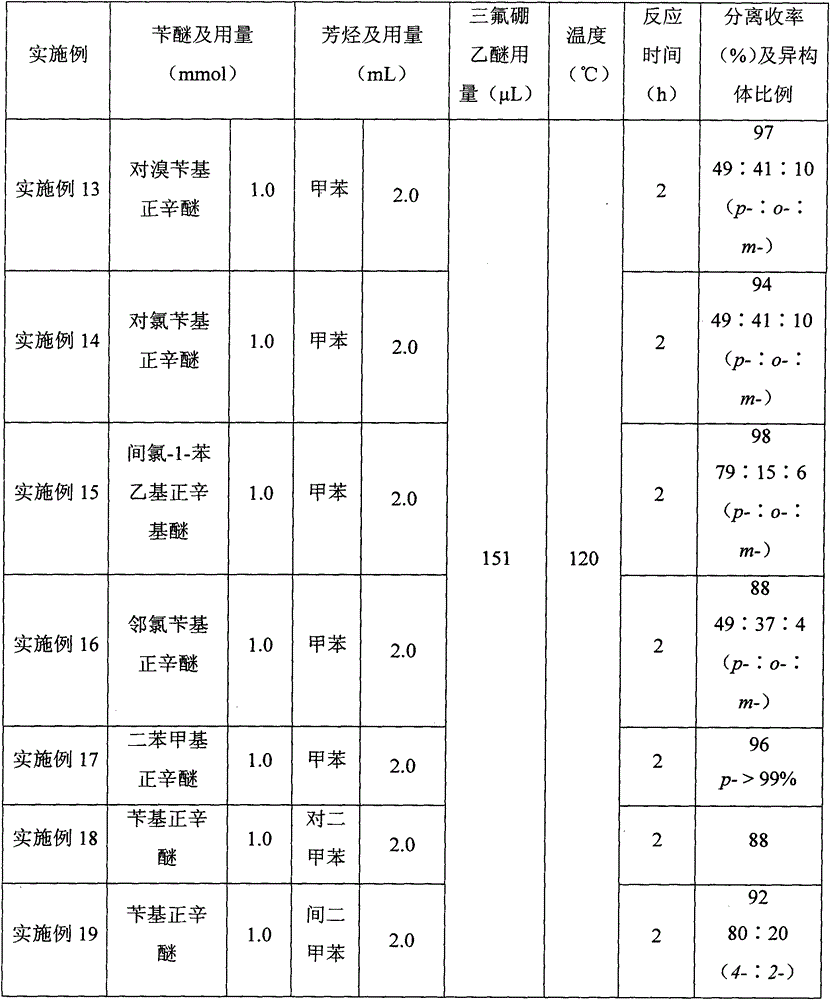
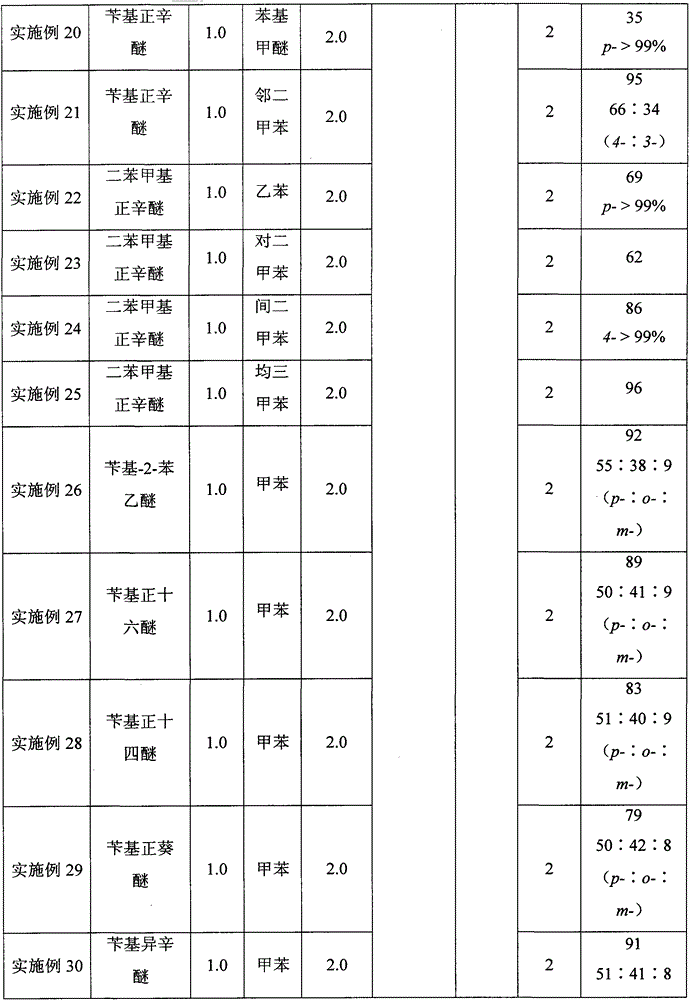
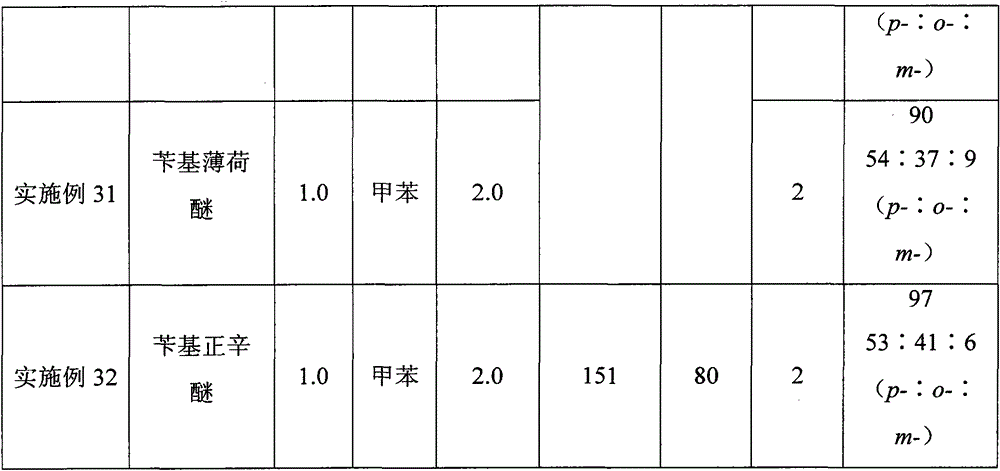
[0024] A kind of synthetic method taking benzyl ether as the diphenylmethane derivative of raw material, its concrete steps are as follows:
[0025] (1) Take benzyl n-octyl ether as the benzylation reagent, boron trifluoride ether as the reaction reagent, and toluene as the raw material and also as the solvent. The ratio is 1:0.2:2. First add benzyl n-octyl ether (220.4mg, 1.0mmol) into the reactor, then add toluene (2.0mL), and then add boron trifluoride diethyl ether (25μL, 0.2 mmol), the addition was completed, and under open conditions, the temperature was raised to 120° C. and continued stirring for 2 hours for benzylation reaction to prepare a reaction solution of benzyltoluene.
[0026] (2) After the first (1) step is completed, the reaction solution of the benzyltoluene prepared in the (1) step is naturally cooled in the air, and the condensing system of the reactor is rinsed with dichloromethane, and the washing liquid is incorporated into the reactor in the reaction...
Embodiment 2
[0029] A kind of synthetic method using benzyl ether as a diphenylmethane derivative of raw material, its concrete steps are the same as embodiment 1, wherein:
[0030] In step (1), benzyl n-octyl ether is used as the benzylation reagent, boron trifluoride ether is the reaction reagent, and toluene is used as the raw material and also as the solvent. : The ratio of toluene to milliliter is 1:2.0:2, first add benzyl n-octyl ether (220.4mg, 1.0mmol) in the reactor, then add toluene (2.0mL), stir, then add boron trifluoride Diethyl ether (252 μL, 2.0 mmol) was added. Under open conditions, the temperature was raised to 120° C. and stirred continuously for 2 hours for benzylation reaction to prepare a benzyltoluene reaction solution.
[0031] In step (2), benzyltoluene (182 mg, yield 99%, p-:o-:m-=49:42:9) was obtained as a colorless transparent liquid.
Embodiment 3
[0033] A kind of synthetic method using benzyl ether as a diphenylmethane derivative of raw material, its concrete steps are the same as embodiment 1, wherein:
[0034] In step (1), benzyl n-octyl ether is used as the benzylation reagent, boron trifluoride ether is the reaction reagent, and toluene is used as the raw material and also as the solvent. : The ratio of toluene to milliliter is 1:1.2:2, first add benzyl n-octyl ether (220.4mg, 1.0mmol) in the reactor, then add toluene (2.0mL), and then add boron trifluoride diethyl ether under stirring (151 μL, 1.2 mmol), after the addition was completed, under open conditions, the temperature was raised to 120° C. and continued to stir for benzylation reaction for 2 hours to prepare a reaction solution of benzyltoluene.
[0035] In step (2), benzyltoluene (181 mg, yield 99%, p-:o-:m-=50:41:9) was obtained as a colorless transparent liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com