Method for preparing chiral polyaniline by protein induction
A chiral polyaniline and protein technology, applied in the fields of chemistry and biochemistry, can solve the problems of complex biological extraction steps, limited application, high price, etc., and achieve the effects of simple, mild, environmentally friendly experimental conditions, low price, and a wide range of sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
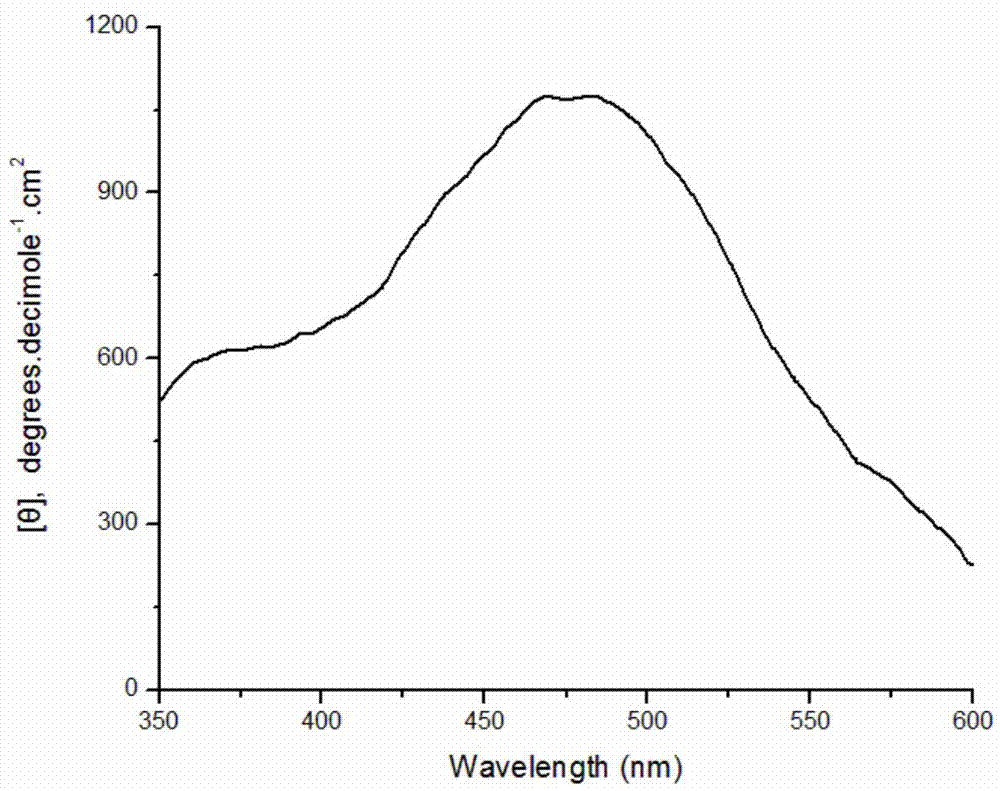
[0028] Weigh 0.0204g of DBSA and dissolve it in 9.7mL of pH2.0 buffer solution; stir well and add 0.125mmol aniline monomer; weigh 10mg of bovine hemoglobin and dissolve it in 200μl distilled water and add it to the system; then add the concentration to 9.823mol 100 μL of hydrogen peroxide solution per L was added to the reaction system in 4 times within one hour. After stirring and reacting for 8 hours, 10 ml of methanol was added to break the emulsion and terminate the reaction. After the product was precipitated, it was collected by centrifugation, and after drying, a dark green powder was obtained with an apparent yield of 138.8%. Characterized by circular dichroism, a positive circular dichroism peak is generated at about 475 nm, which proves that the product is chiral polyaniline, and its molar ellipticity value is 1100 degree cm 2 ·decimole -1 . Circular dichroism spectrum as attached figure 1 shown.
Embodiment 2
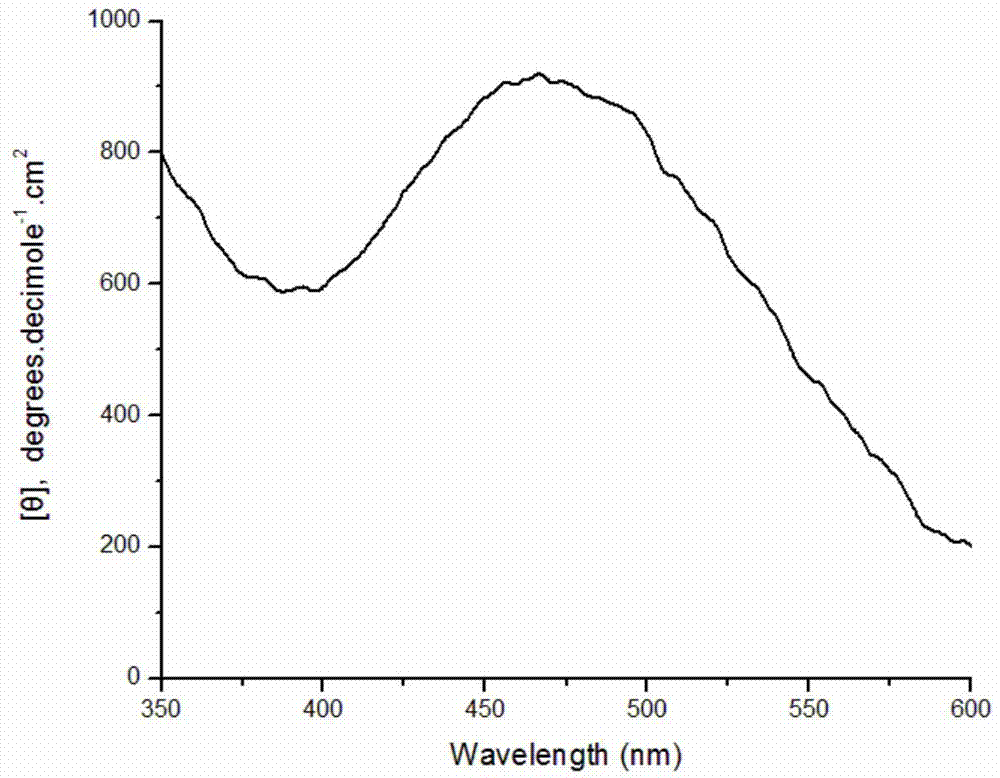
[0030] Weigh 0.0408g of DBSA and dissolve in 9.5mL of pH2.0 buffer solution; stir well and add 0.250mmol aniline monomer; weigh 2mg of bovine hemoglobin and 8mg of bovine serum albumin, dissolve them in 200μl of distilled water and add to the system ; Then 100 μL of hydrogen peroxide solution with a concentration of 9.823 mol / L was added to the reaction system in 8 times within one hour. After stirring and reacting for 12 hours, 10 ml of methanol was added to break the emulsification and terminate the reaction. After the product was precipitated, it was collected by centrifugation, and after drying, a dark green powder was obtained with an apparent yield of 124.3%. Characterized by circular dichroism, a positive circular dichroism peak is generated at about 475 nm, which proves that the product is chiral polyaniline, and its molar ellipticity value is 910 degree cm 2 ·decimole -1 . Circular dichroism spectrum as attached figure 2 shown.
Embodiment 3
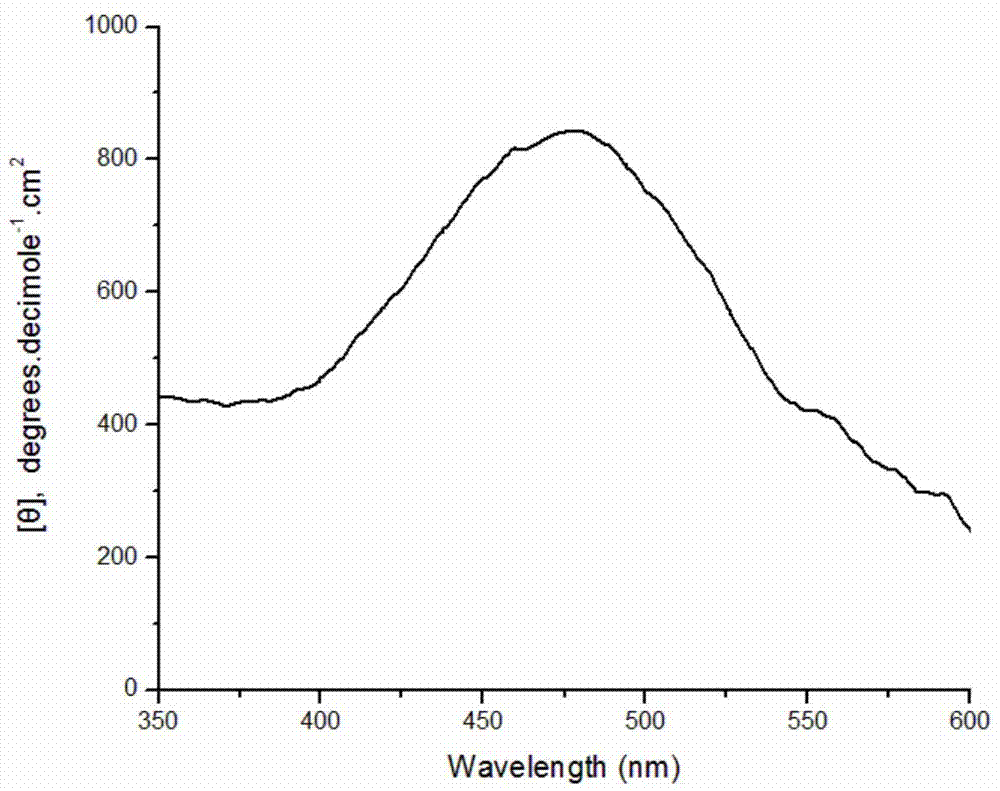
[0032] Weigh 0.0216g of SDS and dissolve in 9.7mL of pH1.0 buffer solution; stir well and add 0.1875mmol of aniline monomer; weigh 10mg of bovine hemoglobin and dissolve it in 200μl of distilled water and add to the system; then add the concentration to 12.5mmol 100 μL of ammonium persulfate solution per L was added to the reaction system five times within one hour. After stirring and reacting for 18 hours, 10 ml of methanol was added to break the emulsification and terminate the reaction. After the product was precipitated, it was collected by centrifugation, and after drying, a dark green powder was obtained with an apparent yield of 121.2%. Characterized by circular dichroism, a positive circular dichroism peak is generated at about 475 nm, which proves that the product is chiral polyaniline, and its molar ellipticity value is 860 degree cm 2 ·decimole -1 . Circular dichroism spectrum as attached image 3 shown
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com