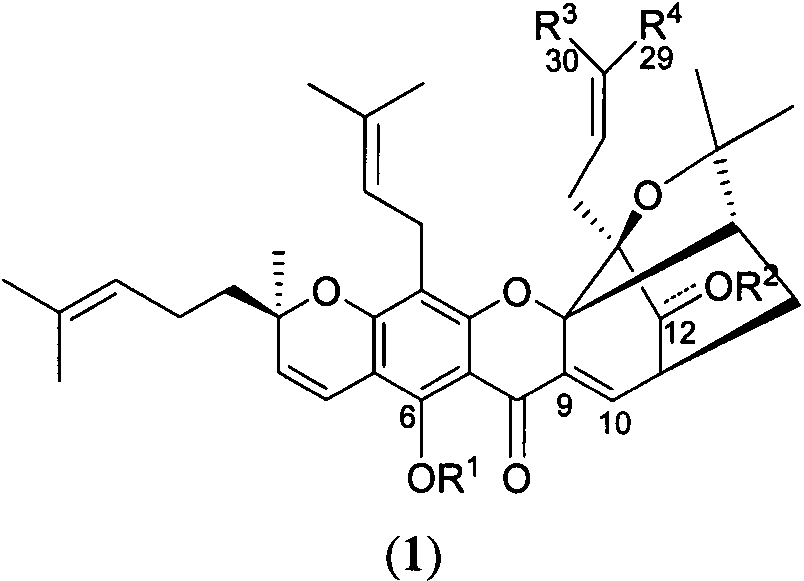
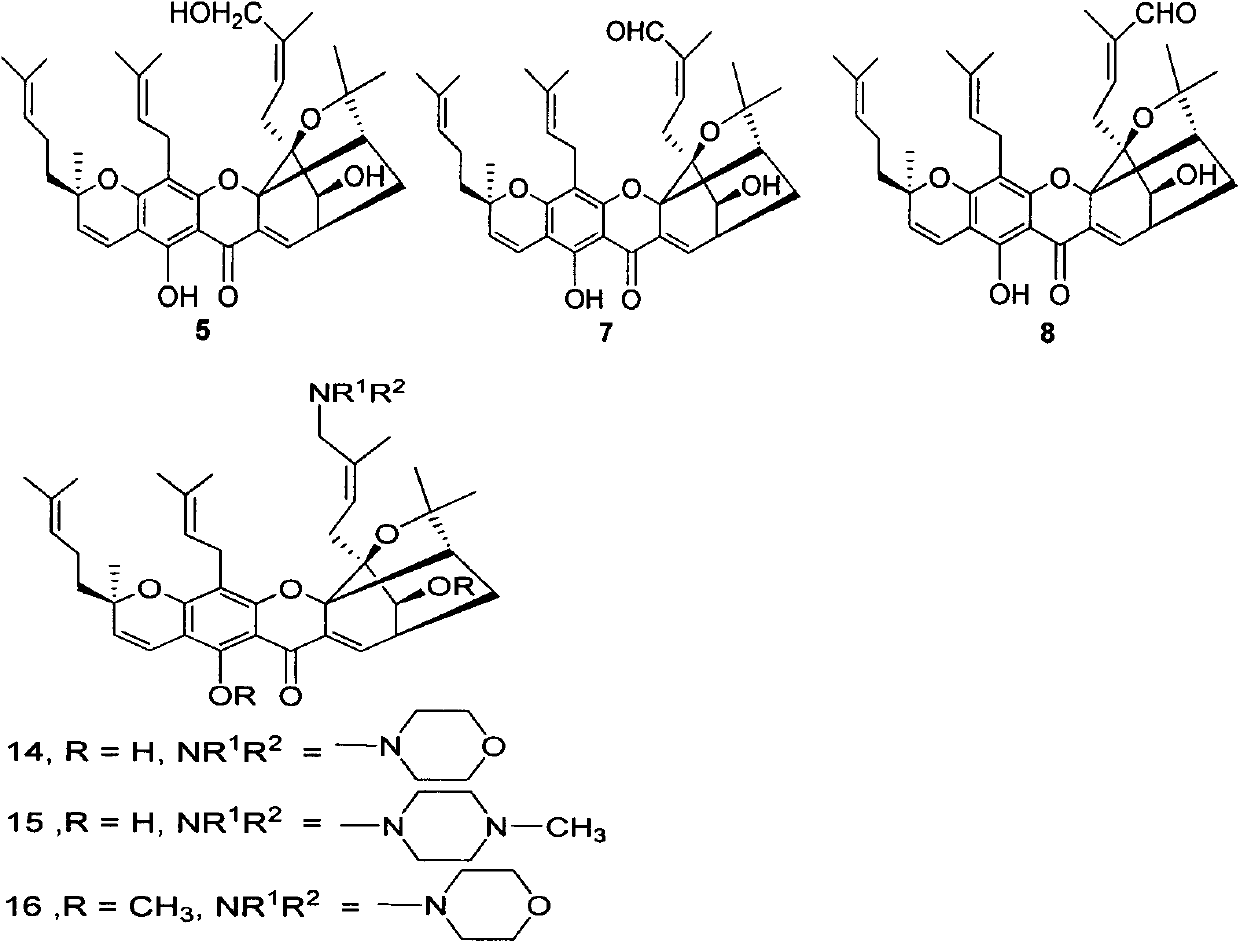
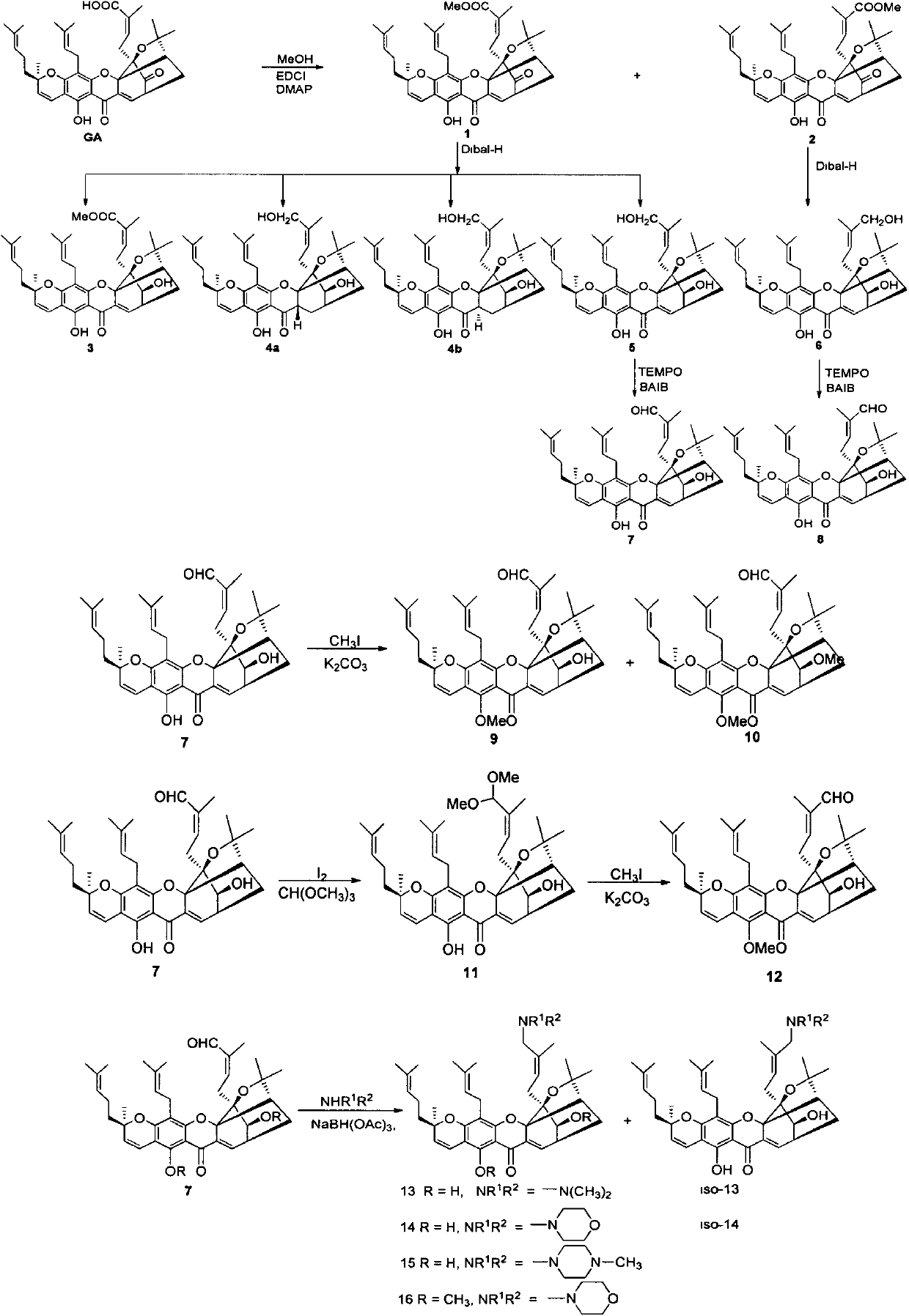
Gambogic acid derivatives as well as preparation method and application thereof
A technology of gambogic acid and derivatives, applied in the fields of gambogic acid derivatives and their preparation and application, can solve the problems of poor water solubility and low bioavailability of gambogic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 methyl gambogic acid and methyl isogambogic acid
[0023] At room temperature, under nitrogen protection, 3g gambogic acid GA (4.78mmol) was dissolved in dichloromethane (50mL), EDCI (1.83g, 9.55mmol, 2eq), DMAP (1.17g, 9.55mmol, 2eq ), finally added methanol 1.93mL (47.8mmol, 10eq), and reacted at room temperature for 2h. Add water to quench the reaction, extract with dichloromethane, combine the organic phases, wash with saturated saline solution, dry over anhydrous sodium sulfate, spin dry, and purify by column chromatography to obtain a yellow solid (methyl esterification product 1 and isomerization methyl ester product 2) .
[0024] Compound 1: orange solid, melting point, 98°C; 1 H NMR (400MHz, CDCl 3 )δ12.85(s, 1H), 7.54(d, J=6.9Hz, 1H), 6.68(d, J=10.1Hz, 1H), 5.93(td, J=7.4, 1.3Hz, 1H), 5.44( d, J=10.2Hz, 1H), 5.10-5.00(m, 2H), 3.48(dd, J=6.8, 4.6Hz, 1H), 3.43(s, 3H), 3.31(dd, J=14.6, 8.0Hz , 1H), 3.15(dd, J=14.6, 5.3Hz, 1H),...
Embodiment 2
[0026] Embodiment 2 Preparation of reduced compound 3 and reduced diol compound 5
[0027] Under nitrogen protection, 1.313g of compound 1 (2.05mmol, leq) was dissolved in dichloromethane, and 5.1mL (6.14mmol, 3eq) of Dibal-H was added dropwise to the solution at -78°C, and supplemented after 3 hours. Add 1.7mL (1eq) Dibal-H, and continue the reaction for 1 hour. Quenched with saturated sodium potassium tartrate solution, extracted with ethyl acetate, combined organic phases, washed with saturated saline solution, dried over anhydrous sodium sulfate, spin-dried, and purified by column chromatography to obtain compounds 3 and 5.
[0028] Compound 3: orange-yellow amorphous solid; 1 H NMR (400MHz, CDCl 3 )δ13.02(s, 1H), 7.65(d, J=7.2Hz, 1H), 6.68(d, J=10.1Hz, 1H), 5.96(t, J=6.8Hz, 1H), 5.43(d, J=10.1Hz, 1H), 5.12-5.00(m, 2H), 3.54(s, 3H), 3.33(dd, J=14.8, 7.8Hz, 1H), 3.29-3.26(m, 1H), 3.23(dd , J=15.4, 4.4Hz, 1H), 3.04(d, J=5.6Hz, 1H), 2.93-2.88(m, 1H), 2.84(dd, J=16.4, 6.4H...
Embodiment 3
[0030] The preparation of embodiment 3 perreduction compound 4a and 4b
[0031] With embodiment 2, difference is that the addition of Dibal-H is 6eq, obtains compound 4a, 4b and 5.
[0032] Compound 4a: orange-yellow amorphous solid, 1 H NMR (400MHz, CDCl 3 )δ12.14(s, 1H), 6.63(d, J=10.1Hz, 1H), 5.42(d, J=10.1Hz, 1H), 5.31-5.22(m, 1H), 5.17(t, J=6.6 Hz, 1H), 5.06(t, J=7.0Hz, 1H), 4.08(d, J=11.7Hz, 1H), 3.64-3.60(m, 2H), 3.34(dd, J=11.7, 7.2Hz, 1H ), 3.28(d, J=6.7Hz, 2H), 2.64(br, 2H), 2.43(d, J=9.6Hz, 1H), 2.36(dd, J=13.6, 10.0Hz, 1H), 2.29(d, J=14.4Hz, 1H), 2.13(dd, J=13.5, 7.0Hz, 1H), 2.05(dd, J=15.7, 7.8Hz, 2H), 1.97(dd, J=12.5, 4.5Hz, 1H), 1.90(d, J=10.2Hz, 1H), 1.84-1.73(m, 12H), 1.69(s, 3H), 1.65(s, 3H), 1.63-1.58(m, 1H), 1.56(s, 3H) , 1.46 (s, 3H), 1.42 (s, 3H) ppm; 13 C NMR (101MHz, CDCl 3 )δ 196.2, 160.5, 158.2, 155.6, 140.4, 131.9, 131.8, 124.7, 123.8, 121.8, 120.2, 115.9, 107.4, 102.2, 101.8, 90.0, 86.5, 82.5, 81.0, 76.5, 64.14, 51.3 , 34.1, 30.3, 29.8, 27.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com