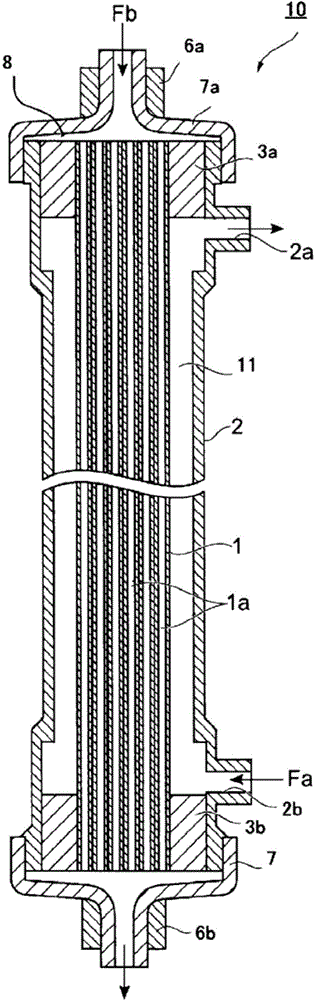
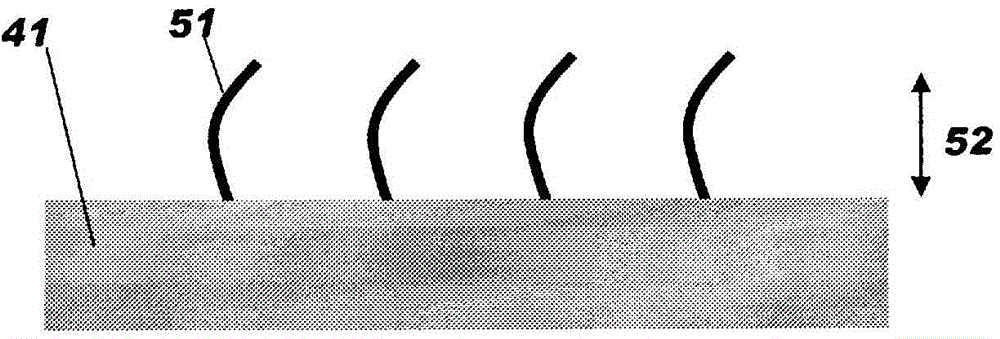
Hollow fiber membrane for blood treatment and hollow fiber membrane-type blood treatment apparatus
A technology of blood treatment and fiber membrane, applied in the direction of fiber chemical characteristics, membrane, membrane technology, etc., can solve the problems of high air permeability of hollow fiber membrane, difficult to distinguish pinholes, etc., achieve high stability, easy start-up, high Effect of Membrane Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0270] In the above-mentioned preparation process of the spinning dope, in order to adjust the content A of the hydrophilic polymer to 3% by mass or more and 10% by mass or less, the dosages of the polysulfone-based polymer and the hydrophilic polymer are adjusted.
[0271] Examples of the common solvent in the spinning dope include solvents such as sulfolane and dioxane, or mixtures of two or more of the above solvents, in addition to the above. In addition, in order to control the pore diameter of the target hollow fiber membrane, additives such as water may also be added to the spinning dope.
[0272] In the process of forming a hollow fiber membrane, as the aforementioned annular slit opening 233 having a double-tube structure, a tube-in-orifice type spinneret is used, and the orifice of the spinneret is The spinning dope is ejected from the tube into the air simultaneously with the hollow inner liquid for coagulating the spinning dope. As the hollow inner liquid, water o...
Embodiment 1
[0403] Prepare a homogeneous spinning dope containing the following ingredients:
[0404] PSf (Solvay Advanced Polymers, L.L.C. manufacture, P-1700) 17 parts by mass
[0405] 4 parts by mass of PVP (manufactured by ISP, K-90)
[0406] 79 parts by mass of dimethylacetamide (hereinafter DMAC).
[0407] Here, PSf represents a polysulfone resin, and PVP represents polyvinylpyrrolidone.
[0408] A 42% by mass aqueous solution of DMAC was used as the hollow inner liquid, and it was ejected from the spinneret together with the spinning dope.
[0409] At this time, the discharge amounts of the spinning dope and the hollow inner liquid were adjusted so that the film thickness after drying was 45 μm and the inner diameter was 185 μm.
[0410] The sprayed spinning dope is immersed in a 60°C coagulation bath composed of water set at 50 cm directly below, and then introduced into a hot air dryer after passing through a coagulation process and a water washing process (washing treatment) ...
Embodiment 2
[0416] Before passing the coating solution into the hollow fiber membrane, pass 120 mL of an aqueous solution consisting of 80 parts by mass of 2-propanol and 20 parts by mass of distilled water, and perform flash evaporation with 0.3 MPa air for 10 seconds.
[0417] Furthermore, the α-tocopherol concentration of the coating solution was set to 0.05% by mass.
[0418] Other conditions were the same as in Example 1, and a hollow fiber membrane blood processing device was obtained.
[0419] In this hollow fiber membrane type blood processing device, the amount of fat-soluble vitamin (α-tocopherol) present on the entire surface of the hollow fiber membrane is 0.5 mg per 1 g of the membrane, and the inner surface of the hollow fiber membrane has high hydrophilicity. The presence ratio B of the molecule (PVP) was 46% by mass, and the content A of the hydrophilic polymer (PVP) in the entire hollow fiber membrane was 3% by mass.
[0420] The results obtained by measuring various pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com